 |
|
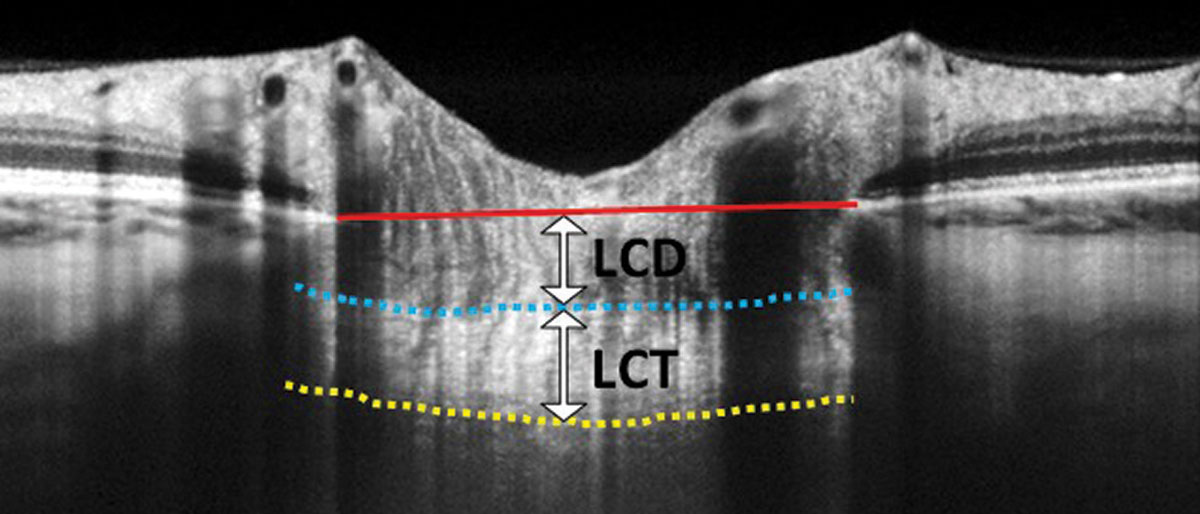
In this EDI-OCT through the optic nerve, the lamina cribrosa thickness (LCT) is measured from the anterior border (blue dashed line) to the posterior border (yellow dashed line) of the lamina cribrosa. Lamina cribrosa depth (LCD) is measured along a perpendicular line from the anterior border of the lamina to a reference line that connects the edges of Bruch’s membrane (red solid line). Photo: Carolyn Majcher, OD. Click image to enlarge. |
One theory of glaucoma pathogenesis suggests there’s an association with the biomechanics of the lamina cribrosa. Since this structure is difficult to study in vivo, researchers turned to the cornea for answers, explaining in a paper published in Journal of Glaucoma that “corneal biomechanics can partially reflect these characteristics [of the lamina cribrosa] since the corneal stroma and sclera are derived from mesoderm.” After conducting a literature review and meta-analysis, they reported that different subtypes of primary open-angle glaucoma (POAG) demonstrated corneal biomechanical differences.
The review included 31 studies with 2,462 POAG patients, 345 ocular hypertension patients (OHT) and 3,281 controls whose corneal biomechanics were measured using either the Ocular Response Analyzer or the Corvis ST. They reported that corneal hysteresis, corneal resistance factor and highest concavity time were lower among POAG patients vs. controls.
Compared with controls, OHT patients demonstrated lower corneal hysteresis, time at second applanation, highest concavity time and radius and deformation amplitude at highest concavity. Corneal resistance factor, time at first applanation and stiffness parameter at first applanation were greater in OHT patients than in controls.
Subgroup analyses revealed that—compared with controls—corneal hysteresis, time at second applanation, length of second applanation and deformation amplitude at highest concavity were lower in high-tension glaucoma patients, while corneal hysteresis, corneal resistance factor, time at first applanation and highest concavity time were lower in normal-tension glaucoma patients.
They concluded that normal-tension glaucoma patients have more deformable corneas, and high-tension glaucoma and ocular hypertension patients have stiffer corneas. “Although the pathology and mechanisms of glaucoma remain controversial, it has been widely accepted that the raised IOP increases the pressure differences across the lamina cribrosa, thus inducing stress and strain on it, eventually leading to compression of the optic nerve head,” the researchers explained in their paper.
To account for glaucomatous changes in those with normal-tension glaucoma, the researchers suggested that the lamina cribrosa in this population may be “abnormally vulnerable to the normal IOP.” They reported that their results confirm this hypothesis, as the “corneal stroma collagen is conjunct with the sclera and lamina cribrosa,” making corneal biomechanics a surrogate of sorts for the properties of the whole eye wall. “Corneal biomechanical measurements could benefit clinical diagnosis,” they wrote in their paper.
Liu M, Zhou M, Li D, et al. Corneal biomechanics in primary open angle glaucoma and ocular hypertension: a systematic review and meta-analysis. J Glaucoma. December 30, 2022. [Epub ahead of print]. |


