Cataracts are the most common cause of visual acuity (VA) loss in the world.1 Most optometrists encounter this condition on a regular basis. Even though patients generally have heard the term “cataract”—derived from the Latin word cataractes, which means “waterfall” and relates to the foamy, hazy appearance of visually significant lens opacities—patients will still have questions about what’s physically happening in their eyes to alter their vision, why these changes are occurring and what to do next.
This article covers five commonly encountered questions cataract patients ask and some appropriate corresponding responses. The way in which we answer these questions and provide guidance can help reduce anxiety and go a long way toward helping patients achieve more successful surgical results.
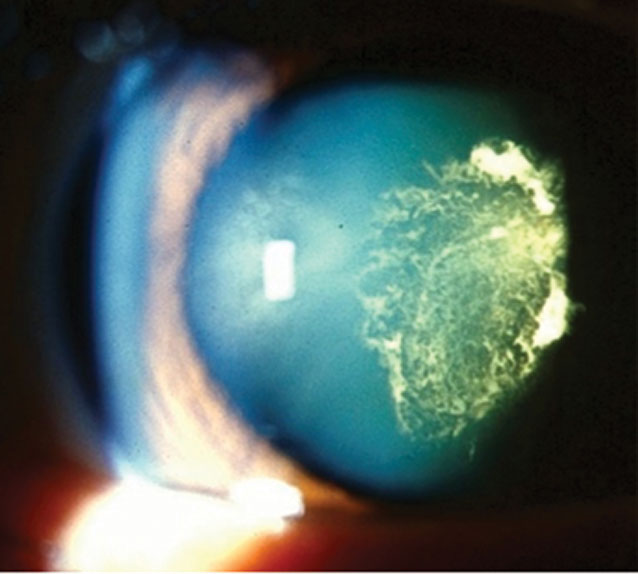 |
| Fig. 1. PSC is more common with diabetes. Click image to enlarge. |
1. “Aren’t I too young for cataracts?”
In one study that reviewed geographical variation in the rate and timing of cataract surgery between US communities, the overall median age for first cataract surgery was 67.7 years, with almost a 20-year variation between regions; median age ranged from 60 to 80.2 While cataracts are most commonly the result of expected age-related changes within the crystalline lens, a variety of conditions may lead to cataract development at an earlier age than anticipated. The study recognized a large variety of contributing factors, some of which are well known, such as comorbidity of diabetes, chronic exposure to UV light and use of corticosteroids. Other associations are less familiar and vary by region, such as exposure to ambient air pollution.2
A different study, conducted in Korea, revealed that individuals, especially females 65 years or older, who were exposed to greater amounts of air pollutants, specifically airborne particulate matter with a diameter of 10µm or less and nitrous dioxide, had a significantly increased hazard ratio and incidence for cataract development.3 Another example of potential environmental effects of toxins/pollutants on cataract development was illustrated by research that found Lake Charles, LA—a major center for petrochemical refining that potentially allows for chronic exposure to naphthalene and other pollutants—had the country’s highest cataract surgery rate.3,4
When a patient believes they are not the “right” age for cataracts but falls into our expected stage for development, reassurance and patient education are recommended. However, when a patient is actually younger than the expected age for lens opacities, it is important to identify potential risk factors or comorbidities by carefully reviewing their history. In addition to known systemic associations, unknown or undertreated conditions may also result in opacification. Reviewing medications closely is valuable, as there are several classes, including but not limited to corticosteroids, phenothiazine and amiodarone, that may disrupt or deposit within the lens. When reviewing family history, a strong presence of cataracts that appears at a younger than anticipated age may also suggest a possible genetic or systemic comorbidity.
Diabetes mellitus (DM) is a common systemic condition that may present with a variety of crystalline lens changes. Patients with DM, especially those who have poorly controlled blood sugar, are more likely to develop cataracts at an earlier age due to accelerated stress on the lens induced by fluctuations in the sorbitol pathway and chronic lens adjustments in response to variable blood sugar levels. All diabetic patients may present with traditional age-related varieties of cataracts but are more likely to have posterior subcapsular cataracts (PSC) and “sugar cataracts” that are cortical in nature (Figure 1). In addition to earlier development, cataracts in patients with DM tend to progress more rapidly.
Wilson’s disease is another metabolic condition that results in lens changes. Individuals with this uncommon autosomal recessive condition may present in the second to fourth decade of life with a rare “sunflower cataract” during active or uncontrolled disease states.5,6 In these patients, the lens may be deposited with copper centrally, which presents as a green-to-brown lens defect surrounded by short stellate processes (small “petals”). However, one study noted that sunflower cataracts are rare in Wilson’s and because there is not a true persistent disruption of the lens and VA is generally not permanently or significantly affected, the term “cataract” might not be the most accurate description for the finding.5,6 While the lens isn’t permanently changed, recognizing this finding in a young patient could save them from permanent complications.
Myotonic dystrophy, a relatively rare systemic condition, is also associated with lens changes. There are several varieties, based in part on chromosomal anomalies that result in type 1 (chromosome 19, CTG repeat) and type 2 (chromosome 3, CCTG repeat), nearly all of which present with early-onset (i.e., younger than 40) characteristic cataracts described as iridescent, cortical “crystals” that are central and polychromatic.7 These pathognomonic lens opacities are known as “Christmas tree cataracts.” Type 2 myotonic dystrophy has also been reported to present with PSC at a relative early age of onset.8
Additional systemic diseases associated with lens changes include but are not limited to Fabry’s disease, atopic dermatitis and neurofibromatosis type 2. Identifying lens anomalies, as well as recognizing associated systemic conditions, may give patients a better understanding of what is happening in their eye as well as their body.
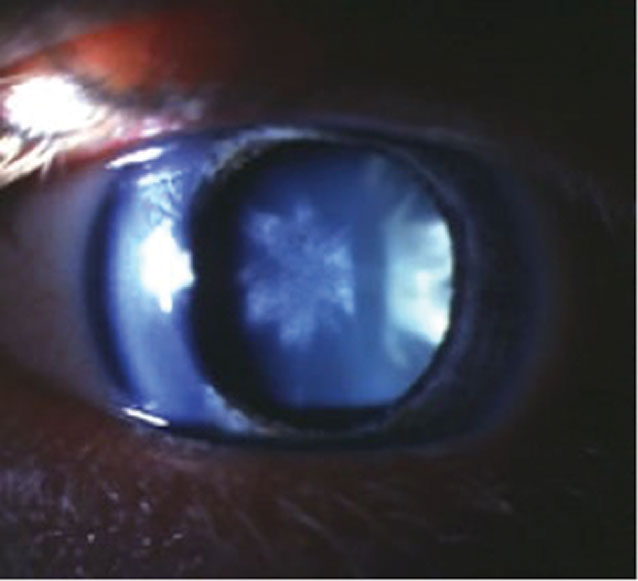 |
| Fig. 2. Rosette lens opacity post-trauma may permanently affect best-corrected VA and is an indication for assessment of other traumatic complications in the eye. Click image to enlarge. |
2. “Is there something I did that made me develop cataracts?”
Ocular history may also be crucial in determining why a patient has visually significant lens changes. The term “complicated cataract” is used to describe opacities that form secondary to a concurrent or chronic ocular comorbidity. As the crystalline lens is avascular and relies on the surrounding ocular fluids to provide support and nutrients for continued, normal lens development, when situations occur where the surrounding material is concurrently exposed to inflammatory cell mediators, lens quality may be compromised. Examples of conditions that are associated with complicated cataracts are most commonly pathologies that cause chronic eye inflammation, such as uveitis associated with juvenile idiopathic arthritis (JIA) or sarcoid disease, retinitis pigmentosa or ocular tumors.
In addition to chronic inflammatory mediators in the eye, acute traumatic events from a variety of sources (e.g., heat, electric shock, irradiation, perforating eye injuries, concussive forces) may appear with a range of cataract presentations. In mechanical trauma, examination may reveal a “rosette” type of cataract with petaloid opacifications radiating from the center of the lens (Figure 2). Rosette lens opacities may be seen in patients who have encountered a blunt trauma to the eye with coup (potentially resulting in a concurrent Vossius ring)-contrecoup injuries.9 The contrecoup injury creates shock waves along a line of traumatic impact, which may generate posterior cortical opacification in the hallmark flower/star pattern.10,11 Patients with visible lens findings after trauma need to be further examined diligently for evidence of other traumatic complications such as disruption of lens zonules, which may occur from axial elongation associated with an injury and significantly complicate future cataract surgery.
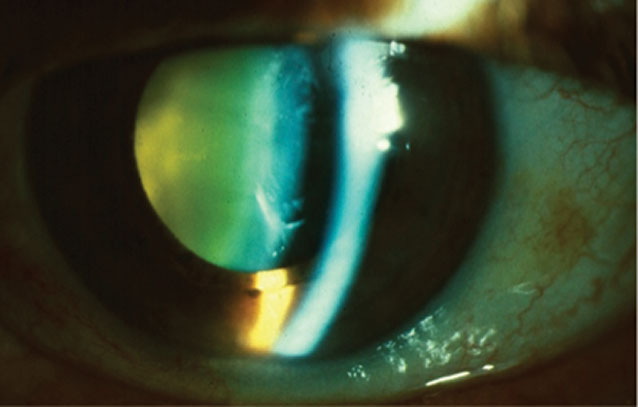 |
| Fig. 3. Crystalline lens yellowing is due to an accumulation of urochrome pigment in the lens. Anterior cortical cataract and nuclear sclerosis are pictured here. Click image to enlarge. |
3. “How did I develop cataracts without any warning signs?”
Prior to decreased VA or symptoms of glare that may result from cataracts, patients might present with early, “soft” signs of changes occurring within the lens. When clinical findings become evident, even prior to changes in best-corrected VA, we should consider using these initial findings as an opportunity to educate the patient on the normal process of lens changes with age and discuss ways to slow progression of visually significant cataract development.
Throughout life, the lens increases in size as both the nucleus and cortex continuously grow. Additionally, the lens may develop an accumulation of urochrome pigment that results in notable color variations on exam, ranging from yellow to brownish red (Figure 3). The increased density of the crystalline lens fibers within the capsule contributes to a change in the index of refraction of the lens and a common myopic shift, often accompanied by a change in astigmatism.
In 2015, a study helped clarify the types of refractive differences that occur by reporting quantified results of 276 subjects after assessing the levels of refractive changes compared with cataract type and severity when classified using the Lens Opacities Classification System III as a grading tool.12,13 The study confirmed that cataracts produced changes in refractive error that were dependent on both the severity and type of opacity, revealing that with higher severity of nuclear sclerosis and PSC, greater myopic shifts and axis rotational changes occurred, and that while cortical cataracts did not produce a myopic shift, there were astigmatic power changes.12
When lens-related refractive changes are identified, the practitioner can educate patients that the shift in prescription might be a “pre-cataract” change. Also, when patients report a need for increased illumination to complete visual tasks (especially at near), this complaint may also serve as an opportunity to make patients aware of early lens alterations. Educating patients about the association between lens changes and more subtle visual refractive symptoms when they occur may prepare them for future vision issues and motivate them to use preventative measures to delay cataract development and progression.
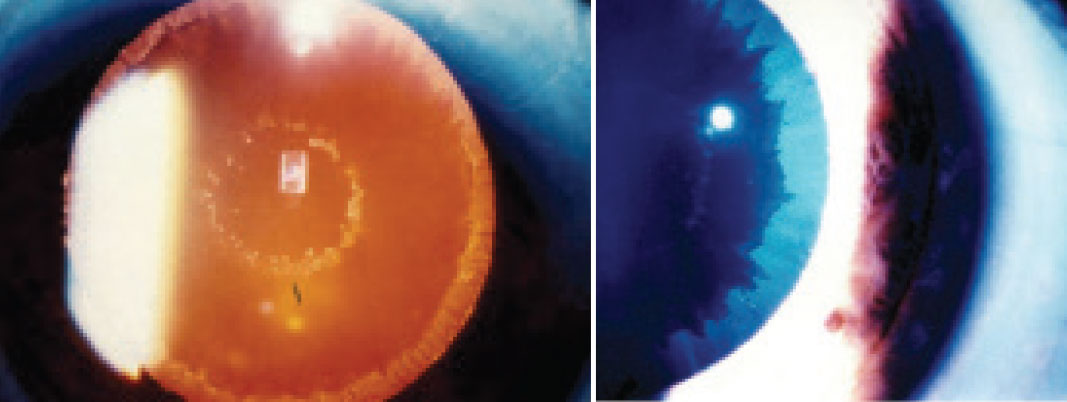 |
| Fig. 4. PXE material on the lens surface can be seen on retroillumination (A) and with white light (B). Click image to enlarge. |
4. “How do I prevent my cataracts from worsening?”
There are a large variety of agents we are exposed to throughout our life—internally and externally—that may contribute to accelerated opacification. Therefore, if we limit exposure to known causative agents, we may be able to decrease the rate at which cataracts become visually significant. Perhaps the most common recommendation to assist with deterrence of lens progression is the use of UV protection in glasses and contact lenses. UV radiation reaches the earth’s surface in the forms of UVB (280nm to 315nm, 1%) and UVA (315nm to 400nm, 99%), penetrating and absorbing into our skin and eyes to result in degenerative changes.
A study analyzing Medicare data for cataract surgery found that after controlling for age, sex, race, income, access to eye care, price of surgery and local practice costs, the strongest predictor of surgery was the person’s latitude of residence.14 Latitude has a direct correlation with the UVB content of sunlight, and data suggests the probability of cataract surgery in the United States increases by 3% for every 1° decrease (i.e., more southerly) in latitude.14 Another study concluded, “Chronic UV effects on the […] lens are cumulative, so effective UV protection of the eyes is important for all age groups and should be used systematically.”15
Encouraging patients to use UV coating on their spectacle lenses, whether tinted or clear, is something ODs can do in the exam room, as well as in the optical. When educating patients on UV, there is value in explaining that UV protection is not necessarily a tint, but rather something that can be added to both clear and colored lenses to be worn whether a day is foggy or clear. E-SPF (Eye Sun Protection Factor) is a relatively new system with a goal of standardizing and quantifying UV protection for lenses that takes into account UV transmission, back reflectance and protection of structures of the eye and adnexal skin tissues.15
Besides encouraging patients to be proactive by using wide-brimmed hats as physical barriers to UV radiation and spectacles or contact lenses that include UV filters, educating those who take steroids on the appropriate use of their medication may further assist in slowing cataract progression. Any chronic use of this drug class may contribute to earlier cataract formation. The well-established association between steroid use and cataracts was further reinforced by a retrospective review of children with JIA who saw their risk of cataracts increase as the number of drops of topical steroids used daily increased.16 The rate dramatically increased from dosing three to four times daily, and the association between steroid use and cataract formation was independent of uveitis activity, severity, duration and relapse.16 Prescribing steroids judiciously and educating patients on associated risks of cataract formation (and intraocular pressure increase) with their steroid-containing medications may assist in slowing visually significant changes.
Smoking is also known to cause morphological and functional changes to the lens. Tobacco modifies ocular capillary perfusion and generates free radicals, thereby decreasing the levels of antioxidants available in the blood, aqueous humor and ocular tissue.17 A 2015 report evaluated the relationship between smoking and cataracts by reviewing 27 studies using causality criteria.18 The review documented a strong association between the two, particularly supporting a causality with nuclear sclerosis.18 In more recent years, traditional cigarette smoking has been accompanied and/or replaced by alternatives such as electronic cigarettes. While there is significantly less literature in regard to electronic cigarettes and cataract development, the association between cataract formation and tobacco use with electronic cigarettes continues to suggest an increased risk of associated lens changes.19
5. “What’s the chance of complications during surgery, and what increases the risk?”
In most cases, optometrists are able to reassure patients that cataract extraction is a safe procedure associated with improvements in daily living, including decreased motor vehicle accidents and falls.20,21 In one study comparing traditional microscope cataract extraction with heads-up cataract surgery (3D), overall complication rates were less than 1% and nearly identical between the traditional group (0.77%) and the 3D group (0.72%).22
Initial steps to assess, manage and reduce potential risks associated with cataract surgery start with the patient’s history. A patient with a history of significant ocular trauma has an increased risk of weakened lens zonules, as well as potential lens subluxation. Additionally, if a patient has a known ocular history of pseudoexfoliation (PXE)—or a history that reveals ocular hypertension and/or glaucoma—perform a careful anterior segment exam to assess for masquerading PXE (Figure 4).
While the exact etiology for PXE is not well understood, multiple factors play a role in the pathogenesis of the disease and how it can complicate cataract surgical outcomes.23 While PXE material may be seen on the anterior lens surface after being deposited from iris vessels with weakened basement membranes, it also deposits on the crystalline lens zonules and weakens them. The zonular compromise may lead to lens subluxation and complicate traditional cataract surgery. Additionally, there are more anterior segment implications associated with PXE material, including iris rigidity and poor mydriasis, which may further complicate cataract extraction and prevent a good overall outcome.
Another risk factor for complications during cataract extraction, which may be identified with a careful history, is the current or past use of tamsulosin and other alpha 1-adrenoreceptors. These medications in particular have been associated with intraoperative floppy iris syndrome (IFIS), a potentially challenging event for even the most prepared surgeons in which iris dilation is compromised and the iris tone is not maintained due to structural changes induced from use of the medication. Since the original association between tamsulosin and IFIS was made, a large number of additional medications and correlated risk factors have been revealed, including gender, age, hypertension and hypertension drugs, benzodiazepines, antipsychotics and finasteride, among others.24
Besides careful histories reviewing ocular status and medications, recognizing patients with posterior polar cataracts (PPC) is also important to reduce the chance of surgical complications. PPC increases the risk for posterior capsular rupture due to preexisting posterior capsular dehiscence. Careful slit lamp exam of the lens of patients with PPC will reveal the presence of a dense, white central opacity on the posterior capsule with multiple concentric layers (bull’s eye-type appearance). These cataracts, which present early in life, may be stationary or progressive, and patients will generally complain of glare. If a patient who needs cataract surgery appears to have a PPC based on general exam findings, further evaluation with anterior segment OCT (AS-OCT) with an emphasis on the posterior lens capsule may provide invaluable insights. A 2018 study looking at 64 eyes of 62 patients that reviewed the effectiveness of AS-OCT for detecting posterior capsule dehiscence found sensitivity and specificity values of 100% and 95%, respectively.25
When PPC is identified prior to cataract extraction, surgeons can implement different techniques to enhance the safety of surgery and reduce the risk for posterior capsular rupture. While there is an inherent degree of risk with any surgical intervention, when potential complications are recognized and precautions are taken, the patient and surgeon can more appropriately set themselves up for an optimal outcome.
Takeaways
In our role as eye care practitioners, we can best serve our patients by thoroughly answering their questions and understanding their concerns. Interestingly, in both of the studies included that reviewed geographical variations in cataract surgery among US communities, when considering the number of ophthalmologists or optometrists in an area, cataract surgery rates were not associated with the number of ophthalmologists but rather with the number of optometrists per 100,000 residents.2,4
Thus, it appears that by better addressing patients’ questions and concerns as part of their cataract care team, we may be able to better assist them in solving their visual shortcomings.
Dr. Tyler is an associate professor at Southern California College of Optometry at Marshall B. Ketchum University. She has no financial interests to disclose.
1. GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9(2):e144-60. 2. Kauh CY, Blachley TS, Lichter PR, et al. Geographic variation in the rate and timing of cataract surgery among US communities. JAMA Ophthalmol. 2016;134(3):267-76. 3. Shin J, Lee H, Kim H. Association between exposure to ambient air pollution and age-related cataract: A nationwide population-based retrospective cohort study. Int J Environ Res Public Health. 2020;17(24):9231. 4. Javitt JC, Kendix M, Tielsch JM, et al. Geographic variation in utilization of cataract surgery. Med Care. 1995;33(1):90-105. 5. Deguti MM, Tietge UJF, Barbosa ER, et al. The eye in Wilson’s disease: sunflower cataract associated with Kayser-Fleischer ring. J Hepatol. 2002;37(5):700. 6. Langwińska-Wośko E, Litwin T, Dzieżyc K, et al. The sunflower cataract in Wilson’s disease: pathognomonic sign or rare finding? Acta Neurol Belg. 2016;116(3):325-8. 7. Ashizawa T, Gagnon C, Groh WJ, et al. Consensus-based care recommendations for adults with myotonic dystrophy type 1. Neurol Clin Pract. 2018;8(6): 507-20. 8. Papadopoulos C, Kekou K, Xirou S, et al. Early onset posterior subscapular cataract in a series of myotonic dystrophy type 2 patients. Eye (Lond). 2018;32(3):622-5. 9. Singh RB, Thakur S, Ichhpujani P. Traumatic rosette cataract. BMJ Case Rep. 2018;11(1):e227465. 10. Tabatabaei A , Kiarudi MY, Ghassemi F, et al. Evaluation of posterior lens capsule by 20-MHz ultrasound probe in traumatic cataract. Am J Ophthalmol 2012;153(1):51-4. 11. Shah MA, Shah SM, Shah SB et al. Morphology of traumatic cataract: does it play a role in final visual outcome? BMJ Open. 2011;1(1):e000060. 12. Díez Ajenjo MA, García Domene MC, Peris Martínez C. Refractive changes in nuclear, cortical and posterior subcapsular cataracts. effect of the type and grade. J Optom. 2015;8(2):86-92. 13. Chylack Jr LT, Wolfe JK, Singer DM, et al. The Lens Opacities Classification System III. Arch Ophthalmol. 1993;111(6):831-6. 14. Javitt JC, Taylor HR. Cataract and latitude. Doc Ophthalmol. 1994-5;88(3-4): 307-25. 15. Behar-Cohen F, Baillet G, de Ayguavives T, et al. Ultraviolet damage to the eye revisited: eye-sun protection factor (E-SPF), a new ultraviolet protection label for eyewear. Clin Ophthalmol. 2014;8:87-104. 16. Thorne JE, Woreta FA, Dunn JP, et al. Risk of cataract development among children with juvenile idiopathic arthritis-related uveitis treated with topical corticosteroids. Ophthalmology. 2020;127(4S):S21-6. 17. Cheng AC, Pang CP, Leung AT, et al. The association between cigarette smoking and ocular diseases. Hong Kong Med J. 2000;6(2):195-202. 18. Kelly SP, Thornton J, Edwards R, et al. Smoking and cataract: review of causal association. J Cataract Refract Surg. 2005;31(12):2395-404. 19. Makrynioti D, Zagoriti Z, Koutsojannis C, et al. Ocular conditions and dry eye due to traditional and new forms of smoking: a review. Cont Lens Anterior Eye. 2020;43(3):277-84. 20. Schlenker MB, Thiruchelvam D, Redelmeier DA. Association of cataract surgery with traffic crashes. JAMA Ophthalmol. 2018;136(9):998-1007. 21. Feng YR, Meuleners LB, Fraser ML, et al. The impact of first and second eye cataract surgeries on falls: a prospective cohort study. Clin Interv Aging. 2018;13:1457-64. 22. Weinstock RJ, Diakonis VF, Schwartz AJ, et al. Heads-up cataract surgery: complication rates, surgical duration, and comparison with traditional microscopes. J Refract Surg. 2019;35(5):318-22. 23. Tekin K, Inanc M, Elgin U. Monitoring and management of the patient with pseudoexfoliation syndrome: current perspectives. Clin Ophthalmol. 2019;13:453-64. 24. Christou CD, Tsinopoulos I, Ziakas N, et al. Intraoperative floppy iris syndrome: updated perspectives. Clin Ophthalmol. 2020;14:463-71. 25. Kumar GP, Krishnamurthy P, Nath M, et al. Can preoperative anterior segment optical coherence tomography predict posterior capsule rupture during phacoemulsification in patients with posterior polar cataract? J Cataract Refract Surg. 2018;44(12):1441-5. |

