 |
A 65-year-old Black female presented for a routine eye examination with a chief complaint of blurred vision, worse in the right eye. She noticed her vision had been declining for at least the past eight months. Her ocular history was remarkable for cataracts, OU. Her systemic history found no hypertension, diabetes or other illness. She denied any past ocular trauma and allergies to medications or other things.
Clinical Findings
Her best uncorrected entering visual acuities were 20/30 OD and 20/25 OS at distance and near with no improvement upon pinhole or refraction. Her external examination was normal and there was no afferent pupillary defect (APD).
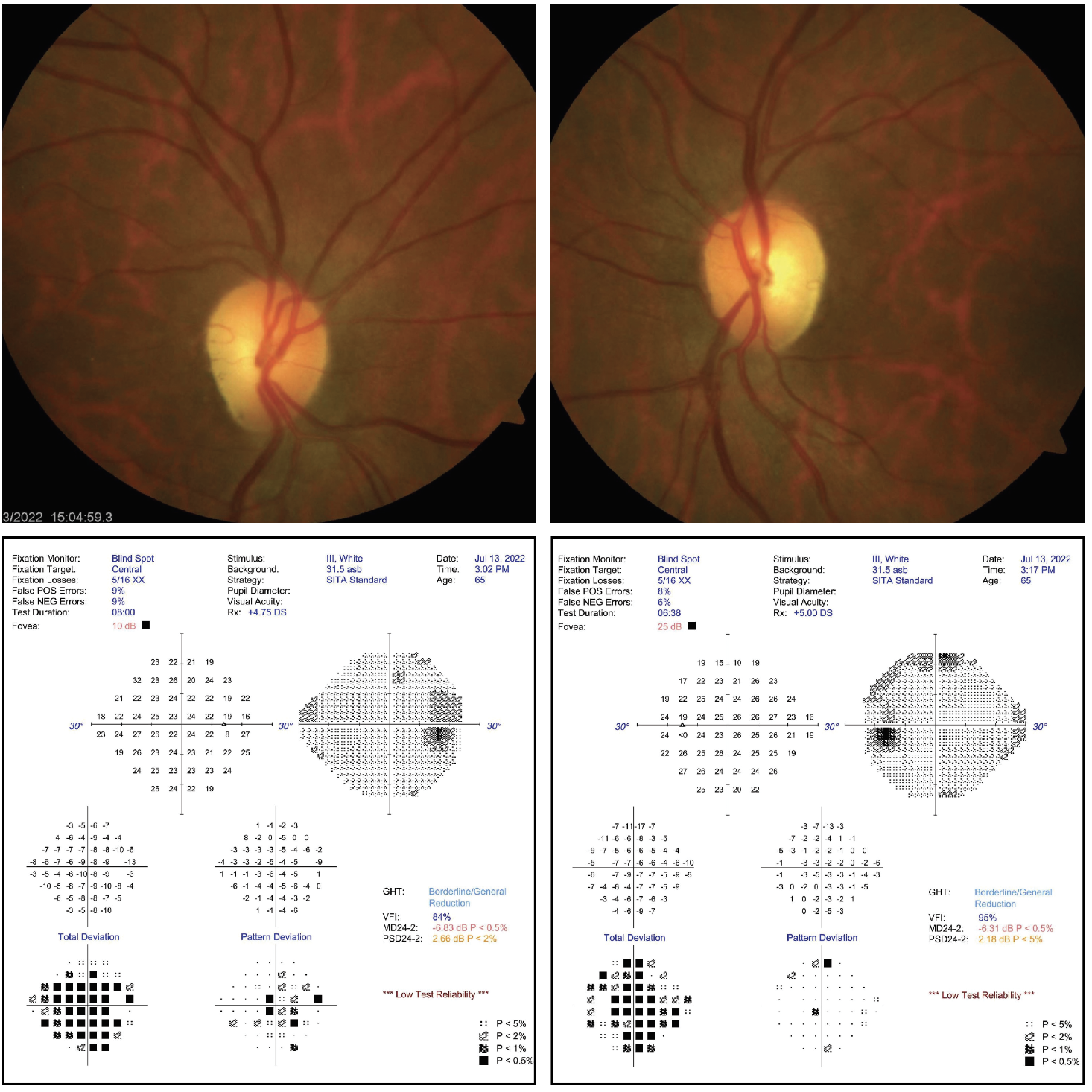
Biomicroscopy uncovered normal tissues and structures OU with Grade II nuclear sclerotic cataracts. Her intraocular pressures measured 16mm Hg by Goldmann applanation tonometry. The pertinent posterior segment findings are demonstrated in the photographs.
Additional testing included color photodocumentation, OCT of both nerves, automated perimetry, red cap color testing, brightness testing and visual fields.
 |
|
Do you notice any correspondence between the fundus photos and the visual field results? If so, what does it signify? Click image to enlarge. |
What would be your diagnosis in this case? What is the patient’s likely prognosis?
Diagnosis
This patient is experiencing bilateral optic disc pallor with slightly increased disc cupping compared to photos taken at her last visit, suggesting non-glaucomatous optic neuropathy rather than glaucoma. The neurosensory retina is composed of a photoreceptor (rod or cone), three neurons (horizontal, bipolar, amacrine cell) and a ganglion cell.1-4 The horizontal, bipolar and amacrine cells serve to create retinal redundancies and receptive fields.4 This allows the axons of over 121 million photoreceptors on average (115 million rods and six million cones) to be paired down to connect with approximately 1.2 million ganglion cells.2-5 The axons of the ganglion cells become the optic nerve.1-6
The neurosensory retina produces graded action potentials when it is exposed to light.1 These impulses require communication with the occipital lobe in the brain to produce vision (Brodmann areas 17, 18 and 19).5 The optic nerve is the conduit.1-5 The ventral stream (often referred to as the secondary visual pathway) moves the impulses from the occipital lobe into the associative areas of the brain, permitting “understanding.”2 While the retinal ganglion cells themselves are unmyelinated, the optic nerve is myelinated.1 The optic nerve is considered an extension of the brain and is covered by the meninges.1
The blood supply for the optic nerve is the ophthalmic artery.7 This vessel arises from the internal carotid artery.7 The ophthalmic artery gives rise to the long and short posterior ciliary arteries (LPCAs and SPCAs), which contribute to the arterial circle of Zinn-Haller.7-10 This structure is considered to be the major arterial supply of the optic nerve and is positioned within the posterior sclera.7 This source supplies the intralaminar region of the nerve.9 The central retinal artery, which arises from the ophthalmic artery, does not contribute to the optic nerve head vasculature proper.8
The optic nerve comingles with the optic stalk during gestation.11-14 The stalk results from the invagination of the optic cup, which arises from the optic vesicles.11-14 A groove that forms along the length of the optic stalk, called the optic fissure, contains the hyaloid artery, which is a branch of the ophthalmic artery.11-13 The hyaloid artery extends from the optic disc to the crystalline lens.11-14 It is most prominent in the ninth gestational week and begins to atrophy starting at seven months, producing the beginnings of the optic cup.11-14
Potential Causes
Optic nerve cupping, or the enlargement of the cup-to-disc ratio, is widely recognized as a feature of glaucoma.3,15 Non-glaucomatous cupping is well known to occur with increased optic nerve pallor.15 There are two principles believed to contribute to acquired optic nerve cupping: prelaminar and laminar thinning.15,16
Laminar thinning occurs secondary to lamina cribrosa deformation and damage to peripapillary scleral connective tissues.15,16 Glaucomatous cupping represents laminar thinning with a sinking optic disc secondary to lamina cribrosa deformation; this leads to nerve fiber layer death with its characteristic correlating functional loss.15 Laminar deformation also results in connective tissue damage.15,16 This causes the optic nerve to be more susceptible to further deformation induced by raised intraocular pressure (IOP).15 Severe disc cupping, which is found in the late stages of glaucoma, will occur if optic nerve atrophy becomes severe.17
Prelaminar thinning occurs when there is loss of ganglion cell axons and/or physical compression.15,16 Prelaminar thinning may be observed in optic neuropathy secondary to compression, ischemia, inflammation, infiltration, toxicity, malnutrition, trauma and genetic or metabolic etiologies.15
Non-glaucomatous optic disc cupping is prelaminar in nature.3,15 The finding is marked by the presence of optic nerve pallor.15 The amount of cupping in these nerves tends to be less pronounced when compared to their glaucomatous counterparts.15 The loss of prelaminar neural tissue increases the cup-to-disc ratio but the result is a shallow form of cupping.6 The most well-recognized causes of non-glaucomatous cupping include compression, inflammation (optic neuritis), ischemia (arteritic and non-arteritic anterior ischemic optic neuropathies), hereditary optic neuropathy (Leber’s hereditary optic neuropathy, dominant optic atrophy) and trauma.3 Non-glaucomatous optic nerve damage may masquerade as glaucomatous cupping.15 Whenever the examiner perceives any portion of the disc as pale, an emergent work up is required to uncover the underlying cause.1-10,15,16
Disc Distress
Optic nerve pallor or optic atrophy represents irreversible damage to the ganglion cell axons.18 Damage resulting in pallor may occur anywhere along the course of the axons from the retina to the lateral geniculate body.18 Primary optic atrophy occurs in the absence of previous disc swelling and appears white with distinct margins and normal retinal blood vessels.1 Secondary optic atrophy develops after there is long-standing swelling of the optic disc.18 These discs are often grayish with indistinct margins.18
Compressive optic neuropathy may present either unilaterally or bilaterally.18 Common signs of compression include slow, progressive vision loss and headaches.19 Lesions located at the orbital apex, superior orbital fissure or cavernous sinus may have associated extraocular motility limitations due to involvement of cranial nerves III, IV or VI.17 Optic nerve compression may induce central or cecocentral visual field defects with an associated APD.18
Lesions of the optic chiasm may reveal a bitemporal hemianopsia.18 The differential list for compressive optic neuropathy is broad, including but not limited to intracranial hypertension, infection (aspergillus), inflammation (thyroid orbitopathy, idiopathic orbital inflammation), vasculopathy (aneurysm, cavernous sinus fistula), trauma (hematoma), neoplasm (glioma, schwannoma), bone tumors/lesions (Paget’s, osteoma) and others such as mucocele and granulomatous meningitis.19
Papilledema is caused by raised intracranial pressure and is usually bilateral.18 Common causes are idiopathic intracranial hypertension (IIH), intracranial space-occupying lesions and meningitis.18 Longstanding papilledema will result in optic pallor, atrophy and possibly “pale cupping.”18 Treatment of compressive optic neuropathy depends on the etiology.18,19
Inflammatory disease of the optic nerve may initially present with disc edema.18 A common cause for inflammatory optic neuropathy is optic neuritis.15 Such patients often present with painful subacute vision loss.15 In-office examination may reveal visual field loss, color vision defects and an APD of the affected eye.19
Optic neuritis can either be idiopathic or, more likely, secondary to a demyelinating condition such as multiple sclerosis (MS) or the neuromyelitis optica spectrum of disorders.15 Optic neuritis associated with MS typically affect patients aged 20 to 40 years.17 Resultant optic nerve pallor is often shown to be temporal.15
Other inflammatory causes include infections such as tuberculosis and syphilis. Autoimmune-related conditions such as systemic lupus erythematosus and sarcoidosis are also common offenders.18 Appearance of inflammation observed on the MRI may help differentiate amongst autoimmune, infectious and granulomatous etiologies.20
Neuropathy Types
Ischemic optic neuropathy (ION) is the result of infarction of any part of the optic nerve.18,21 Patients often present with sudden unilateral painless vision loss.18,21 Optic disc pallor may appear as diffuse or sectoral.18 Sectoral pallor often presents with an associated altitudinal field defect.18 ION may be categorized as anterior or posterior.15 Anterior ION by definition is characterized by disc edema.15,21 The much rarer posterior ION, in contrast, may look unremarkable in an acute setting.15 Pallor in posterior ION likely indicates chronic ischemia.15 ION is also categorized as arteritic (giant cell arteritis) and non-arteritic (presumed embolic).15
Non-arteritic anterior ischemic optic neuropathy (NAION) is the most common acute optic neuropathy in patients aged over 50 years.22 An estimated incidence of NAION among this age group is around 2.3 to 10.2 per 100,000 population, with about 1,500 to 6,000 new cases in the US each year.22 It is thought to be the result of hypoperfusion of the anterior portion of the optic disc via occlusion of the short posterior ciliary arteries (SPCA), but the exact pathophysiology is still unknown.22,23 Risk factors include advanced age, hypertension, diabetes, smoking, nocturnal hypotension and small optic discs (disc at risk).21,22
Common signs and symptoms of NAION include acute unilateral painless vision loss.23 APD may be present in the affected eye.23 Visual fields often reveal an arcuate or altitudinal defect (frequently inferior field).23 Funduscopy will reveal disc edema with occasional peripapillary flame-shaped hemorrhages.23 Diffuse or segmental pallor of the disc develops after four to six weeks.23 Examination of the fellow eye almost always reveals a disc at risk (optic nerve small in stature).23 Risk of second-eye involvement ranges from 15% to 25%.22 Bilateral involvement is extremely rare.23 Diagnosis of NAION is difficult due to lack of confirmatory tests.23 Treatment and prevention of NAION is controversial.23 There is currently no general consensus due to the unknown nature of its pathophysiology.23
Anterior arteritic ION (AAION) is most often the result of giant cell arteritis (GCA).15 This is an immune-mediated systemic granulomatous vasculitis where the lumen of the affected vessels is forced closed by invading multi-nucleated giant cells.24 Typical age of occurrence is over 50 years and the condition is most common in caucasions.22,23 The blood vessels most affected by GCA are medium to large in size.24 Classic presentations include headache, jaw claudication and polymyalgia rheumatic (PR).23,24 In AAION, the SPCA are affected.25,26 Patients often present with sudden unilateral vision loss with associated APD and significant visual field defect.21 Funduscopy often reveals a pale or “chalky-white” swollen disc.21,24
AAION is considered an ophthalmic emergency, given the high risk of second-eye involvement.24 Immediate evaluation for GCA is imperative.21 Temporal artery biopsy is the gold standard for diagnosis, but studies have shown questionable yields.27 Blood work for GCA includes erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). These tests, when positive or negative together, have a combined sensitivity of up to 99.2% for GCA.21,27
When GCA is suspected, systemic intravenous (methyl prednisone) or oral (prednisone) corticosteroids should be administered immediately even in the setting of waiting for test results so as to minimize losses in the affected eye and prevent vision loss in the fellow eye.24
The most common hereditary optic neuropathies are Leber’s hereditary optic neuropathy (LHON) and dominant optic atrophy (DOA).28 LHON is a maternally inherited mitochondrial disease that is highly associated young adult men.29-31 Patients often present with painless, subacute vision loss.29-31 Typically, only one eye is affected initially with the second eye developing symptoms a few months to a few years after the first.31 The most common age of onset is during the second and third decades of life.30 Males are four to five times more likely to be affected than females.30
The disease is primarily diagnosed using clinical findings.29 Funduscopy will reveal disc edema with hyperemia, retinal telangiectasia and increased vascular toruosity.30 This is often followed by optic atrophy with temporal pallor.29,30 Visual field testing will reveal central or cecocentral scotomata.29,30 Color vision will be reduced.29,30 A maternal family history of vision loss is also helpful in diagnosis.29 Definitive diagnosis is made via molecular genetic testing assaying for the mitochondrial DNA mutation.29-31 The most common genes affected are MT-ND1, MT-ND4 and MT-ND6.31
Therapy for LHON is controversial at best.28 In 2015, the European Medicines Agency (EMA) approved idebenone (Raxone/Catena, Santhera Pharmaceuticals) for treatment of LHON under exceptional circumstances.29 Clinical trials of idebenone have indicated better long-term visual prognosis with at least 18 to 24 months of treatment.32
DOA is a neuro-ophthalmic disorder characterized by bilateral degeneration of the optic nerves.33 It leads to insidious vision loss that starts within the first decade of life.33 It primarily targets the retinal ganglion cells and axons that form the optic nerve.33 Patients typically experience moderate vision loss with associated central or paracentral visual field defects and reduced color vision.33 Funduscopy reveals optic nerve pallor and atrophy.33 Diagnosis is usually made during early childhood in the setting of unexplained vision loss and optic nerve pallor.33 Usually, there is a family history of DOA.33 Optical coherence tomography (OCT) will reveal non-specific thinning of the retinal nerve fiber layer.33 Electroretinogram (ERG) and visual evoked potentials (VEP) may also reveal abnormal function of the retinal ganglion cells and axons.33 Molecular diagnosis can also be made via identification of the OPA1 or OPA3 gene mutation.33 Vision loss may progress slowly during puberty until adulthood.33
Prognosis
In this case, in the setting of otherwise normal IOP, the primary concern was the bilateral disc pallor and its underlying cause. Our examination revealed equivocal afferent functionality with slightly decreased vision, possibly secondary to her cataracts. Visual field testing demonstrated diffuse, non-specific depression with no obvious glaucomatous changes. A referral was made to neuro-ophthalmology to assess our diagnosis and to rule out additional work up.
Neuro-ophthalmology agreed with our observations, ordering a battery of testing to rule out non-glaucomatous sources of optic neuropathy. Ultimately, she was diagnosed with old “unspecified” bilateral optic atrophy. No additional treatment or intervention was recommended. She will be carefully monitored in both the primary care and neuro-ophthalmology services biannually.
Dr Gurwood thanks Sam Kim, OD, for contributing this case.
Dr. Gurwood is a professor of clinical sciences at The Eye Institute of the Pennsylvania College of Optometry at Salus University. He is a co-chief of Primary Care Suite 3. He is attending medical staff in the department of ophthalmology at Albert Einstein Medical Center, Philadelphia. He has no financial interests to disclose.
1. Yazdankhah M, Shang P, Ghosh S, et.al. Role of glia in optic nerve. Prog Retin Eye Res. 2021;81(3):100886. 2. Sing N, Anderson S, Townsend J. The normal optic nerve head. Optom Vis Sci. 2000;77(6):293-301. 3. Jonas J, Schmidt A, Müller-Bergh J, et al. Human optic nerve fiber count and optic disc size. Invest Ophthalmol Vis Sci. 1992;33(6):2012-8. 4. Zapp S, Nitsche S, Gollisch T. Retinal receptive-field substructure: scaffolding for coding and computation. Trends Neurosci. 2022;45(6):430-445. 5. Kawachi J. Brodmann Areas 17, 18, and 19 in the Human Brain: An Overview. Brain Nerve. 2017;69(4):397-410. 6. Waisberg E, Micieli J. Neuro-ophthalmological optic nerve cupping: an overview. Eye Brain. 2021;13(12):255-268. 7. Charlick M, Das J. Anatomy, Head and Neck, Internal Carotid Arteries. [Updated 2021 Jul 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK556061/ 8. Hayreh, S. Blood supply of the optic nerve head and its role in optic atrophy, glaucoma, and oedema of the optic disc. The British journal of ophthalmology. 1969;53(11): 721-48. 9. Jonas J, Holbach L, Panda-Jonas S. Peripapillary arterial circle of Zinn-Haller: location and spatial relationships with myopia. PLoS One. 2013;8(11):e78867. 10. Makino S, Takezawa M, Sato Y. A case of incomplete central retinal artery occlusion associated with short posterior ciliary artery occlusion. Case Rep Ophthalmol Med. 2013;2013:105653. 11. Azzam D, Bordoni B. Embryology, Optic Fissure. [Updated 2022]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. 12. Rosen D, Mahabadi N. Embryology, Optic Cup. [Updated 2022]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. 13. Sheth J, Sharma A, Chakraborty S. Persistent hyaloid artery with an aberrant peripheral retinal attachment: A unique presentation. Oman J Ophthalmol. 2013;6(1):58-60. 14. Tawfik H, Dutton J. Embryologic and fetal development of the human orbit. Ophthalmic Plast Reconstr Surg. 2018;34(5):405-421. 15. Waisberg E, Micieli J. Neuro-ophthalmological optic nerve cupping: an overview. Eye Brain. 2021;2021(13):255-268. 16. Burgoyne C. The morphological difference between glaucoma and other optic neuropathies. J Neuroophthalmol. 2015;35 Suppl 1(1):S8-S21. 17. Shin Y, Uhm K. Acase of optic nerve atrophy with severe disc cupping after methanol poisoning. Korean Journal of Ophthalmology. 2011;25(2):146-150. 18. Osaguona V. Differential diagnoses of the pale/white/atrophic disc. Community Eye Health. 2016;29(96):71-74. 19. Rodriguez-Beato F, De Jesus O. Compressive optic neuropathy. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. 20. Bennett J. Optic Neuritis. Continuum (Minneap Minn). 2019;25(5):1236-1264. 21. Patel H, Margo C. Pathology of ischemic optic neuropathy. Arch Pathol Lab Med 2017;141(1): 162–166. 22. Shir Yen W, Yathavan S, Ramli M, et al. Bilateral sequential non-arteritic anterior ischemic ptic Nenuropathy (NAION). Cureus. 2021;13(11):e19408. 23. Atkins E, Bruce BB, Newman N, Biousse V. Treatment of nonarteritic anterior ischemic optic neuropathy. Surv Ophthalmol. 2010;55(1):47-63. 24.Hayreh S. Giant cell arteritis: Its ophthalmic manifestations. Indian J Ophthalmol. 2021;69(2):227-235. 25. Patil P, Karia N, Jain S, Dasgupta B. Giant cell arteritis: a review. Eye Brain. 2013 9;5:23-33. 26. Levine S, Hellmann D. Giant cell arteritis. Current Opinion in Rheumatology: 2002;14(1): 3-10. 27. Mandura R. Giant cell arteritis presenting as uilateral arteritic anterior ischemic optic neuropathy. Cureus. 2021;13(7):e16653. 28. Newman, N., Biousse, V. Hereditary optic neuropathies. Eye. 2004;18, 1144–1160 (2004). 29. Carelli V, Carbonelli M, de Coo I, et al. International consensus statement on the clinical and therapeutic management of Leber hereditary optic Nneuropathy. Journal of Neuro-Ophthalmology. 2017;37(4):371-381. 30. Yu-Wai-Man P, Chinnery P. Leber hereditary optic neuropathy. 2000 [Updated 2021]. In: Adam M, Mirzaa G, Pagon R, et al., editors. GeneReviews [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2022. 31. Manickam A, Michael M, Ramasamy S. Mitochondrial genetics and therapeutic overview of Leber's hereditary optic neuropathy. Indian J Ophthalmol. 2017;65(11):1087-1092. 32. Catarino C, von Livonius B, Priglinger C, et al. Real-world clinical experience with idebenone in the treatment of Leber hereditary optic neuropathy. J Neuroophthalmol. 2020;40(4):558-565. 33. Lenaers G, Hamel C, Delettre C, et al. Dominant optic atrophy. Orphanet J Rare Dis. 2012;9(7):46. |

