 |
Three recent patients, all coming later in the day as emergencies, underscored a need for cooperative care to ensure the best outcome.
The first was a 64-year-old woman who noticed an obscuration in her right lower visual field. She was 20/400 in that eye from a longstanding macular scar, but she knew something was different. Threshold perimetry revealed an inferior arcuate defect and a slightly pale and swollen optic disc in that eye. When questioned, she mentioned some mild intermittent headache controlled by oral analgesics, loss of seven pounds over the past four weeks, mild loss of appetite and some mild malaise and fatigue. Otherwise, she felt well.
The second was a 63-year-old woman who had been sent from a local emergency room because she had woken up that morning completely blind in her left eye. When she presented, she stated that she had improved somewhat from complete blindness in that eye but felt that the vision was still terrible. Vision testing and perimetry revealed 20/30 acuity and a markedly constricted visual field with only central fixation remaining. She reported frontal and occipital headaches for several months. She also stated that her appetite was nil and that she had lost 15lbs over the past several months. She said that she had been generally unwell since contracting COVID several months earlier and reported fatigue, malaise and lethargy. Funduscopically, she had a pale, swollen optic disc.
The third patient was an 88-year-old man who had noted a sudden change of vision in his right eye five days previously. His visual acuity was counting fingers at 4ft with a dense inferior arcuate scotoma in that eye. Like the previous patients he manifested a pale, edematous optic disc in that eye. Upon detailed questioning, he denied any headache, jaw claudication, weight loss, appetite loss and had no constitutional symptoms to speak of.
Each of these three patients shared the same diagnosis of anterior ischemic optic neuropathy (AION) and based upon age and symptoms were suspected of having specifically arteritic AION and giant cell arteritis (GCA). Each was sent (or in the case of patient #2, sent back) to an emergency room with information about the presumptive diagnosis, and recommendations to obtain an erythrocyte sedimentation rate, C-reactive protein and platelet count. Additionally, I provided my cell phone number with instructions to call back with the results and consultation.
 |
|
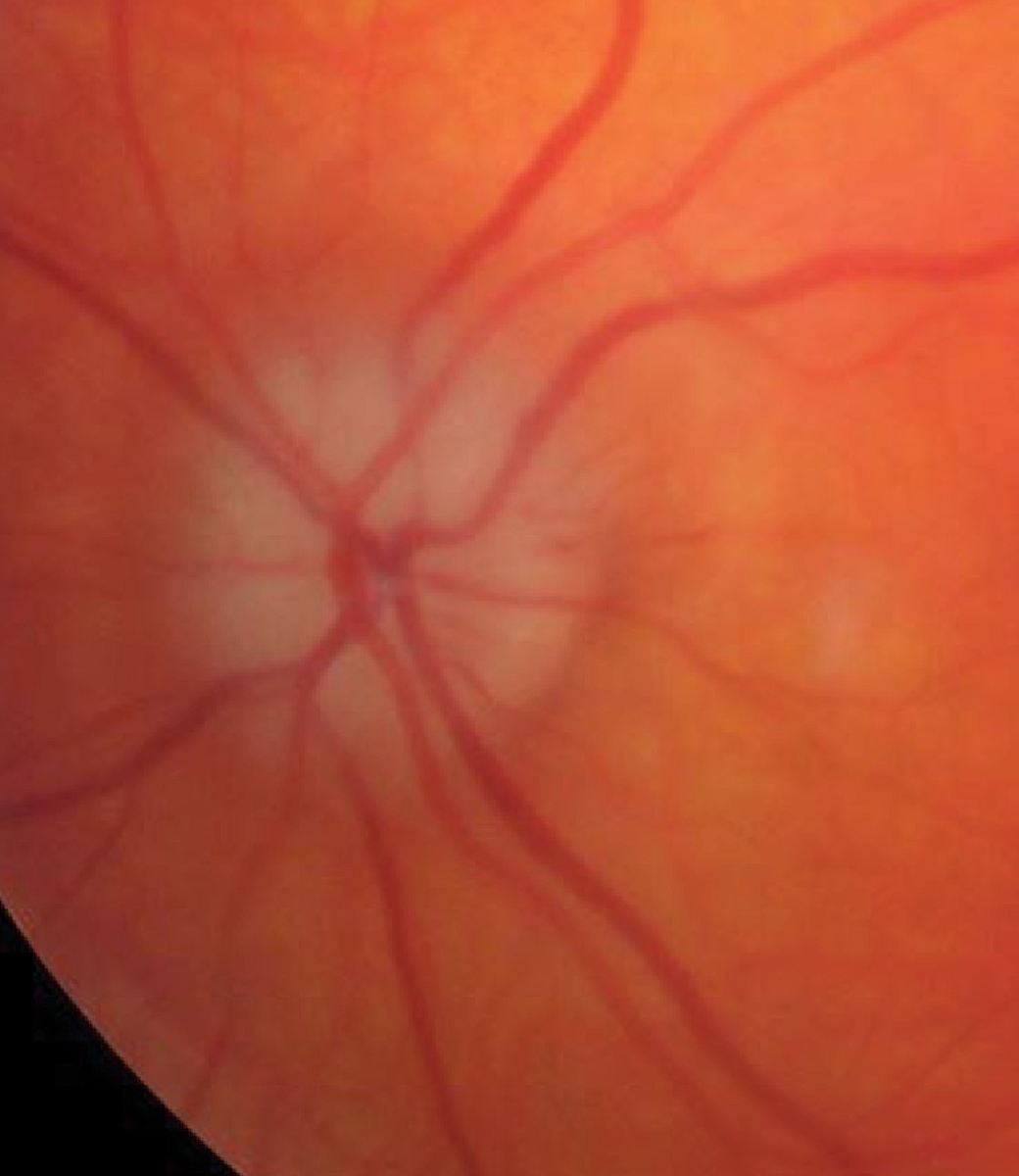
A pale, swollen disc in arteritic AION. Click image to enlarge. |
Discussion
One of the true emergencies in all of eye care is a patient suffering vision loss from GCA, coming in the form of ischemic optic neuropathy (anterior or posterior) or retinal artery occlusion. In many cases what begins as unilateral devastating vision reduction quickly progresses to bilaterality and total visual disability for the patient.1,2
Patients suffering from GCA are elderly, with a mean age of 71 at presentation.3 This condition is generally considered only after 50, with women somewhat more likely to develop it.4
There are a multitude of systemic manifestations occurring in GCA including malaise, weight loss and anorexia, headache (typically in the temporal or occipital region), pulseless and indurated temporal arteries, night sweats, tongue necrosis and oral ulceration, dental abscess, scalp pain, scalp necrosis and jaw claudication when eating. There is also head and neck swelling, anemia, depression, mental disturbance, neck pain, low grade fever, transient ischemic attack and stroke, proximal myalgia, persistent flu-like illness, chronic pharyngitis, vertigo, muscle aches, cardiac arrhythmia, congestive heart failure and myocardial infarction.5-13 There are patients who will show no or minimal systemic symptoms of this disease.
GCA is a granulomatous inflammation of medium- and large-sized arteries that have a defined internal and external elastic lamina.5 The interleukin (IL)-6 pathway is upregulated in GCA. There is cellular infiltration of the muscular wall of these vessels by T lymphocytes, macrophages, histiocytes, plasma cells and multinucleate giant cells.14,15 The resultant inflammation fragments the vascular walls and leads to collapse of the vessel lumen with resultant ischemia. Conclusive diagnosis of GCA occurs through temporal artery biopsy. However, the procedure is invasive, and false-negative results can confound the diagnosis. More recently, temporal artery ultrasound has been increasingly used as an accurate, noninvasive method for assisting in disease diagnosis.16
Therapy Protocol
Treatment is high-dose systemic steroids, either oral or in-patient intravenous infusion.17-19 Patients with vision loss or other ocular complications should receive four daily infusions of 250mg of methylprednisolone for three days, which is best done on an in-patient basis.20 After the initial infusion regimen, the patient is released with instructions to continue the oral steroid until they can be seen by a rheumatologist.
For oral administration, the initial prednisolone dose is 60mg to 80mg/day. It should be reduced in weekly steps of 5mg to 10mg until 20mg/day, and by 2.5mg until 10mg/day. Dose reduction is then 1mg/month below 10mg/day, depending on symptoms, erythrocyte sedimentation rate and C-reactive protein. Suppression of the disease usually takes months to years, leaving patients and physicians to cope with complications of long-term steroid use such as ulcers and gastrointestinal bleeding, osteoporosis, increased risk of heart disease, diabetes, decreased bone density, increased risk of infections, thin skin, easier bruising and slower healing of wounds.
Actemra (tocilizumab, Genentech) is a biologic IL-6 receptor antagonist, used as a steroid-sparing therapy to maintain disease remission in patients with GCA.21 It has been used in conjunction with oral steroids to better suppress the disease long term. Patients receiving tocilizumab had superior disease remission at one year compared with the steroid-only taper. Challenges to adjunctive use of tocilizumab include expense, formulary limitations and uncertainly when to finally stop use in disease suppression.
In each of the three patients presented here, I used local hospital emergency departments to get testing, evaluation and management initiated quickly.
In each case, a typical trend was followed. Shortly after discharging the patient, I received a call from an emergency room physician who relayed positive test results and then asked what protocol for therapy should be followed. We discussed the patient and the disease entity, and I recommended that each patient be admitted for intravitreal (IV) steroid therapy. I recommended 250mg solumedrol every six hours for 12 doses in-patient with discharge with 80mg prednisone PO until consultation with a rheumatologist. I also helped them to arrange a temporal artery ultrasound with a local vascular surgeon skilled in the technique. In each case, several hours later I received telephone calls from the admitting hospitalists who also wanted to discuss the patients’ cases and verify the orders for IV steroids and the proper dosing.
What I found in all cases is that the emergency physicians and hospitalists all had excellent knowledge of GCA but lacked confidence and experience and were extremely grateful that I could walk them through the steps, steroid dosing, route of administration, need for ancillary testing, and discharge plans. In each case, the patients maintained vision in their remaining fellow eye, and one had some visual improvement in the involved eye.
As optometrists, we are unlikely to be administering IV steroids and admitting patients for GCA treatment, but knowledge of treatment for this disease is critically important when we are working collaboratively with other physicians to obtain the best possible outcome for our patients.
Dr. Sowka is an attending optometric physician at Center for Sight in Sarasota, FL, where he focuses on glaucoma management and neuro-ophthalmic disease. He is a consultant and advisory board member for Carl Zeiss Meditec and Bausch Health.
1. Hayreh SS, Podhajsky PA, Zimmerman B. Ocular manifestations of giant cell arteritis. Am J Ophthalmol. 1998;125(4):509-20. 2. Wein FB, Miller NR. Unilateral central retinal artery occlusion followed by contralateral anterior ischemic optic neuropathy in giant cell arteritis. Retina. 2000;20(3):301-3. 3. Nordberg C, Johansson H, Petursdottir V, et al. The epidemiology of biopsy-proven giant cell arteritis: special reference to changes in the age of the population. Rheumatology (Oxford). 2003;42(4):549-52. 4. Salvarani C, Gabriel SE, O’Falon WM, et al. The incidence of giant cell arteritis in Olmstead County, Minnesota: apparent fluctuations in a cyclic pattern. Ann Intern Med 1995;123(3):192-4. 5. Rahman W, Rahman FZ. Giant cell (temporal) arteritis: an overview and update. Surv Ophthalmol. 2005;50(5):415-28. 6. Zachariades N, Skoura C, Spanou A, et al. Temporal arteritis: report of a case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(2):192-7. 7. Gurwood AS, Brilliant R, Malloy KA. The enigma of giant cell arteritis: multidisciplinary management of two cases. J Am Optom Assoc. 1998;69(8):501-9. 8. Liozon E, Ouattara B, Portal MF, et al. Head-and-neck swelling: an under-recognized feature of giant cell arteritis. A report of 37 patients. Clin Exp Rheumatol. 2006;24(2 Suppl 41):S20-5. 9. Gonzalez-Gay MA, Garcia-Porrua C, Llorca J, et al. Visual manifestations of giant cell arteritis. Trends and clinical spectrum in 161 patients. Medicine (Baltimore). 2000;79(5):283-92. 10. Nordborg E, Nordborg C. Giant cell arteritis: strategies in diagnosis and treatment. Curr Opin Rheumatol. 2004;16(1):25-30. 11. Liozon E, Loustaud V, Fauchais AL, et al. Concurrent temporal (giant cell) arteritis and malignancy: report of 20 patients with review of the literature. J Rheumatol. 2006;33(8):1606-14. 12. Manetas S, Moutzouris DA, Falagas ME. Scalp necrosis: a rare complication of temporal arteritis. Clin Rheumatol. 2007;26(7):1169. 13. Biebl MO, Hugl B, Posch L, et al. Subtotal tongue necrosis in delayed diagnosed giant-cell arteritis: a case report. Am J Otolaryngol. 2004;25(6):438-41. 14. Albert DM, Searl SS, Craft JL. Histologic and ultrastructural characteristics of temporal arteritis. The value of the temporal artery biopsy. Ophthalmology. 1982; 89(10):1111-26. 15. Ashton-Key M, Gallagher PJ. Surgical pathology of cranial arteritis and polymyalgia rheumatica. Baillieres Clin Rheumatol 1991; 5(3):387-404. 16. Scheurer RA, Harrison AR, Lee MS. Treatment of vision loss in giant cell arteritis. Curr Treat Options Neurol. 2012;14(1):84-92. 17. Serling-Boyd N, Stone JH. Recent advances in the diagnosis and management of giant cell arteritis. Curr Opin Rheumatol. 2020;32(3):201-7. 18. Gonzalez-Gay MA, Martinez-Dubois C, Agudo M, et al. Giant cell arteritis: epidemiology, diagnosis, and management. Curr Rheumatol Rep. 2010;12(6):436-42. 19. Schmidt J, Warrington KJ. Polymyalgia rheumatica and giant cell arteritis in older patients: diagnosis and pharmacological management. Drugs Aging. 2011;28(8):651-66. 20. Schmidt WA. Current diagnosis and treatment of temporal arteritis. Curr Treat Options Cardiovasc Med. 2006;8(2):145-51. 21. Unizony SH, Dasgupta B, Fisheleva E, et al. Design of the tocilizumab in giant cell arteritis trial. Int J Rheumatol. 2013;2013:912562. |

