 |
Managing complex ocular cases through the application of clinical knowledge, high-quality data and experience can maximize patient outcomes— but when we ourselves become the patient, we can pass along the perspective gained and nuanced features learned to our colleagues and form the basis for delivering true person-centered care.
As a retina patient myself, I’ve received periodic intravitreal bevacizumab injections followed by anterior chamber paracentesis for a number of years for the treatment of chronic low-grade cystoid macular edema following a complex retinal detachment repair (a tale for another time). As an experienced intravitreal anti-VEGF injection patient, I know what to expect post-injection: ocular surface irritation and tearing and burning for 15 to 45 minutes, followed by visual improvement and relief in ocular comfort throughout the day.
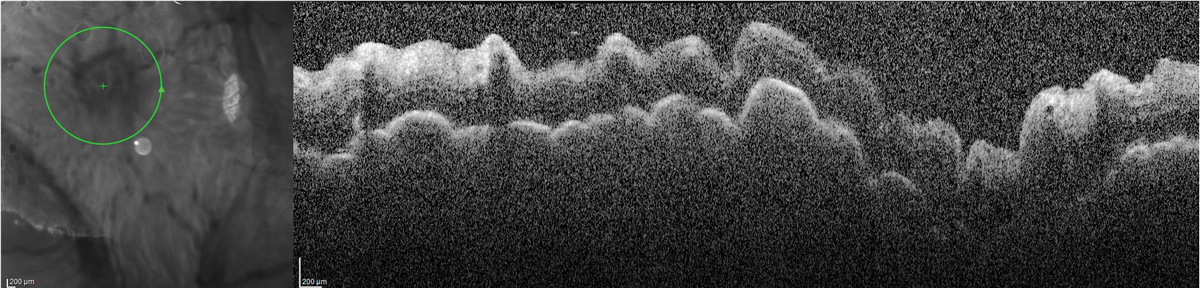
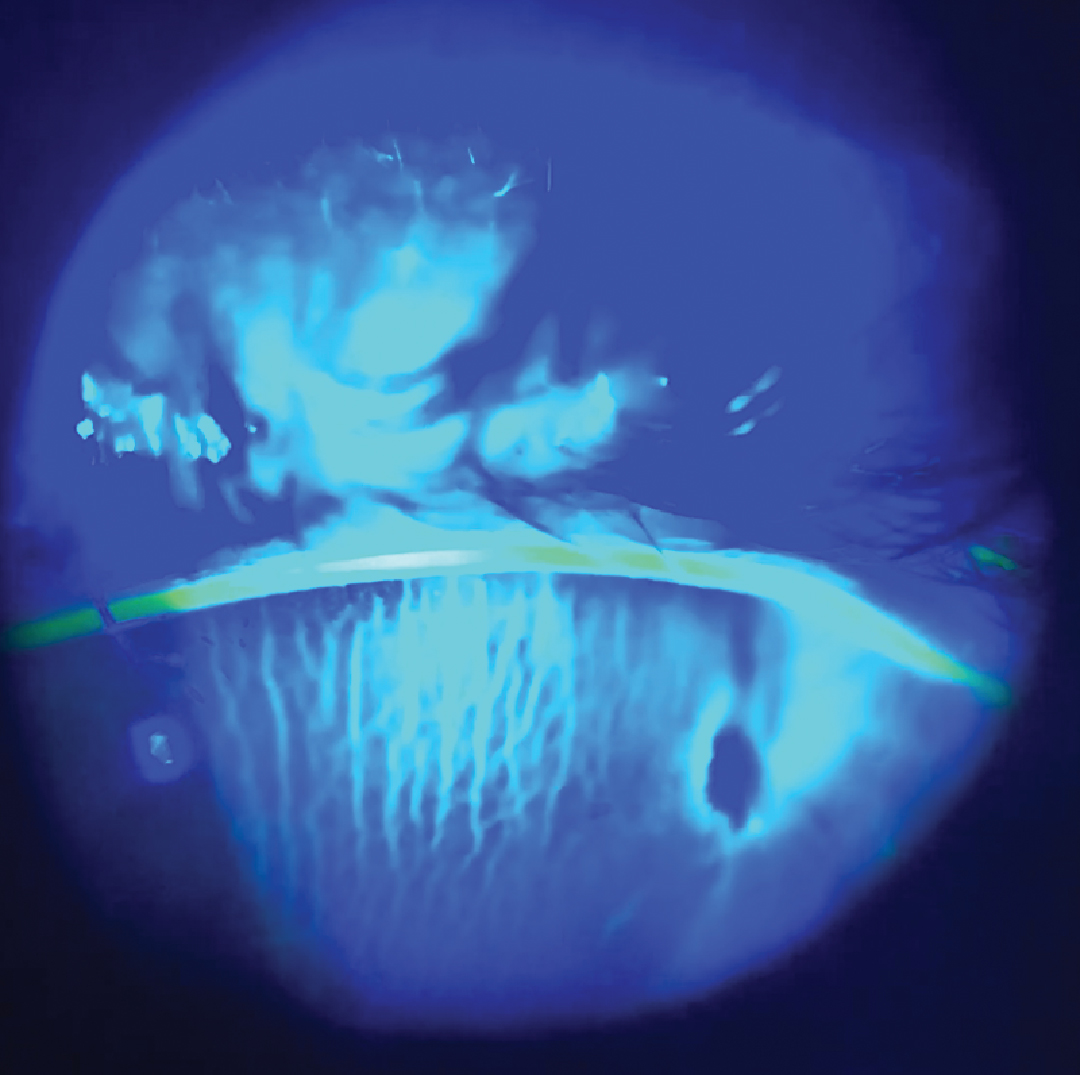
So, the post-procedure experience of significant unilateral tearing and the feeling of a very “full” tear film, in the absence of redness or irritation after the resolution of surface-related symptoms, was something new—and concerning. By the sixth hour following the injection and paracentesis, now back in the retina surgeon’s office, my intraocular pressure (IOP) was 2mm Hg, and my vision had dropped from 20/40 to 20/400 with a positive Seidel sign—not from the scleral site where the intravitreal injection was performed but from the peripheral corneal paracentesis site, with the presence of chorioretinal folds in the posterior pole.
 |
|
Peripapillary OCT demonstrating chorioretinal folds in the setting of hypotony. Click image to enlarge. |
Background
Treating retinal pathology with intravitreal injections has transformed long-term outcomes for patients with sight-threatening disease associated with exudation and neovascularization. While adverse events associated with intravitreal injections are most often centered on vision-threatening complications such as rare inflammatory and infectious events, the most common adverse effect, transient elevated IOP, and its potential long-term consequences on optic nerve health, are often left out of the discussion.
Transient elevated intraocular pressure is an expected phenomenon following intravitreal injection and is reported to typically range from 28mm Hg to 55mm Hg but has been reported to be as high as 87mm Hg. The proposed mechanism for elevated IOP is increased volume of fluid within the vitreous cavity, so higher-volume intravitreal therapies, including the newly approved Syfovre (pegcetacoplan, Apellis Pharmaceuticals) 0.15mg/0.1mL, may be expected to have a greater impact on post-procedure IOP in comparison with most intravitreal anti-VEGF agents, which have a volume of 0.05mL.1-5
While the long-term impact of this immediate, sudden IOP spike on retinal vascular circulation and optic nerve function, especially in individuals with a high burden of treatment for chronic retinal pathology, has yet to be completely understood, the expected impact on the risk of development or progression of glaucomatous optic neuropathy has led treating surgeons to explore additional options to minimize or prevent the IOP rise.1,6-10.
Anterior chamber paracentesis has been cited as an effective means of mitigating the IOP spike either immediately prior to or following intravitreal injection.1,7-9 In a series of 1,661 patients who received anterior chamber paracentesis following intravitreal injection of either bevacizumab or triamcinolone acetonide, the mean post-injection IOP was 9mm Hg with a median 210µL of aqueous removed.7 Importantly, no instances of endophthalmitis, wound leak or negative outcome were observed in any of the cases.7
The risk of hypotony following anterior chamber paracentesis appears to be very rare, with one case series of five eyes of five patients described in the literature.9 Hypotony risk has been suggested to be associated with serial paracentesis due to long-term degradation of corneal integrity with repeat trauma, which may make cases more complex to manage in comparison with persistent wound leak following cataract surgery due to “coring” of corneal tissue and resulting poor wound closure.9
Previously reported cases of hypotony following anterior chamber paracentesis associated with intravitreal injections resolved with a range of treatments of escalating invasiveness, which range from bandage contact lens placement, pressure patching and corneal sealant use to corneal suturing.9
 |
|
Positive Seidel test and anterior corneal |
Swift Solutions
After a persistent wound leak from the paracentesis site was determined, topical moxifloxacin for prophylaxis of infection was instilled in-office, and a tight pressure patch was placed over my left eye for two days. One disposable eye patch was folded in half and placed under a second patch adhered with three strips of very tightly stretched surgical tape.
For pressure patch placement, paper-based tape with limited stretch is not preferred, as it makes it difficult to apply the patch tightly. Following removal of the pressure patch, difluprednate 0.05% was instilled every three to four hours, moxifloxacin was instilled 0.5% TID and cyclopentolate 1% was instilled once daily.
A tight pressure patch was placed each night and removed in the morning with the goal of keeping the eye closed with limited motility under the patch.
My vision subjectively improved rapidly after the first 48 hours and was 20/60 with a fully formed anterior chamber, closed wound leak and IOP of 28mm Hg after five days.
At five days, the chorioretinal folds had resolved, but a subtle, focal serous detachment remained. The topical steroid and antibiotic were discontinued, and intraocular pressure, visual acuity and macular anatomy all returned to baseline by 14 days.
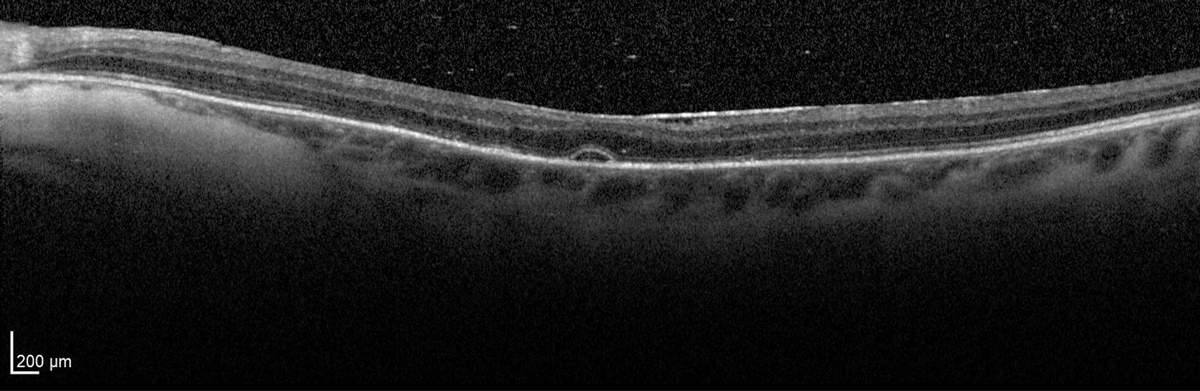 |
Resolution of chorioretinal folds with remaining focal serous detachment at day five. Click image to enlarge. |
Takeaways
Early recognition and aggressive treatment, with a combination of medical therapy and a tightly applied pressure patch, led to a quick recovery and excellent visual outcome.
Everybody has “something.” When that something overlaps with the conditions that we diagnose and manage in our own patients, that personal experience and intimate understanding of management considerations—as well as the ability to share that experience with colleagues—ultimately elevates the care that we continue to provide to our patients.
I remain so grateful to Timothy Murray, MD, Aaron Gold, OD, and the team at Murray Ocular Oncology and Retina for their ongoing exceptional care and constant pursuit to improve treatment outcomes.
Dr. Steen is an assistant professor at Nova Southeastern University College of Optometry where she serves as director of the Glaucoma Service, coordinator of the Primary Care with Emphasis in Ocular Disease Residency and teaches courses in glaucoma and ocular pharmacology. Her financial disclosures include Bausch & Lomb, Santen, Ocuphire and Carl Zeiss Meditec.
1. Hoguet A, Chen PP, Junk AK, et al. The effect of anti-vascular endothelial growth factor agents on intraocular pressure and glaucoma: a report by the American Academy of Ophthalmology. Ophthalmology. 2019;126(4):611-22. 2. Grzybowski A, Told R, Sacu S, et al; Euretina Board. Update on intravitreal injections: Euretina expert consensus recommendations. Ophthalmologica. 2018; 239(4):181-93. 3. Bracha P, Moore NA, Ciulla TA, et al. The acute and chronic effects of intravitreal anti-vascular endothelial growth factor injections on intraocular pressure: a review. Surv Ophthalmol. 2018;63(3):281-95. 4. Hollands H, Wong J, Bruen R, et al. Short-term intraocular pressure changes after intravitreal injection of bevacizumab. Can J Ophthalmol. 2007;42(6):807-11. 5. Gismondi M, Salati C, Salvetat ML, et al. Short-term effect of intravitreal injection of ranibizumab (Lucentis) on intraocular pressure. J Glaucoma. 2009;18(9):658-61. 6. Shah SM, Boopathiraj N, Starr MR, et al. Risk, prevalence, and progression of glaucoma in eyes with age-related macular degeneration treated with intravitreal anti-vascular endothelial growth factor injections. Am J Ophthalmol. 2022;243:98-108. 7. Bach A, Filipowicz A, Gold AS, Latiff A, Murray TG. Paracentesis following intravitreal drug injections in maintaining physiologic ocular perfusion pressure. Int J Ophthalmol. 2017;10(12):1925-7. 8. Saxena S, Lai TY, Koizumi H, et al; International Pharmacokinetic Collaboration. Anterior chamber paracentesis during intravitreal injections in observational trials: effectiveness and safety and effects. Int J Retina Vitreous. 2019;5:8. 9. Shah AP, Sisk RA, Foster RE. Complications of serial anterior chamber paracentesis for increased intraocular pressure after intravitreal injections. Retin Cases Brief Rep. 2022;16(2):136-40. 10. Khodabande A, Zarei M, Khojasteh H, et al. The effect of acute rises in intraocular pressure after intravitreal bevacizumab injection on the peripapillary retinal nerve fiber layer thickness and the role of anterior chamber paracentesis. J Curr Ophthalmol. 2021;33(1):12-6. |

