 |
As a clinician who spends each clinic day in the trenches, and in keeping with my idea of penning columns for fellow doctors in the trenches, sometimes the ancillary issues with rendering good patient care need to be discussed. Whether we are managing a case of glaucoma, even straightforward cases such as this one, or more complex issues like neuro-ophthalmic disorders, coordinating care for our patients is part and parcel of every day in clinic. While many of us don’t actually do all the coordinating of the behind-the-scenes pieces to the puzzle of delivering patient care, such as wading through the pre-authorization process for a particular medication, we do spend considerable time communicating with other physicians about the particular patient we are dealing with, such as with their primary care internist when a patient has diabetes.
In glaucoma care, the majority of our “behind-the-scenes” time is spent dealing with pre-authorizations for medications, coordinating interventional glaucoma surgery, also coordinating a surgical plan for our glaucoma patients who need cataract surgery and discussing the potential for incorporating various minimally invasive glaucoma surgery (MIGS) devices such as iStent inject. Occasionally, though, there is a curveball thrown at us that consumes even more of our time, and that or our ancillary staff. This is one of those situations.
Case
 |
|
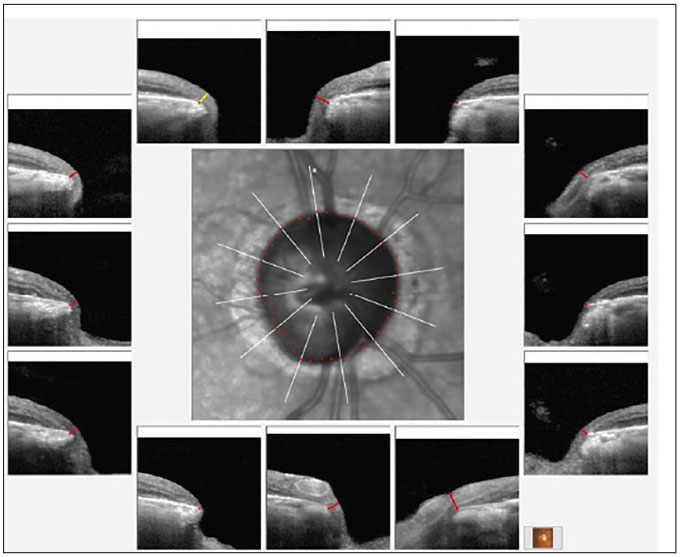
Fig. 1. Bruch’s membrane opening minimum rim width (BMO-MRW) overview of the right neuroretinal rim. Note the eroded temporal rim, along with notching of the inferotemporal segment. Click image to enlarge. |
This pleasant 79-year-old African American male presented as a new patient about two years prior with advanced glaucoma OD and moderate glaucoma OS. His initial visit was to establish care with me and establish if I could help him see better with his right eye in particular, as his previous provider was not able to improve his vision. Current medications included travoprost HS OU and Cosopt (dorzolamide hydrochloride and timolol maleate ophthalmic, Théa Pharma) BID OU. Systemic medications included lisinopril, metformin and 81mg aspirin daily with no reported allergies to medications.
At the initial visit, pressures were 10mm Hg OD and 14mm Hg OS by applanation. Pachymetry readings were 501µm OD and 509µm OS. Best-corrected visual acuities were 20/60 OD and 20/30+ OS. Pupils were ERRLA with a +1 APD OD, extraocular movements were full in all positions of gaze and the anterior segments were unremarkable with deep open chamber angles, as the patient was pseudophakic. Through dilated pupils, his intraocular lenses were well-centered OU; the posterior capsule OD was opened and that of the left was clear and intact. Bilateral posterior vitreous detachments were present.
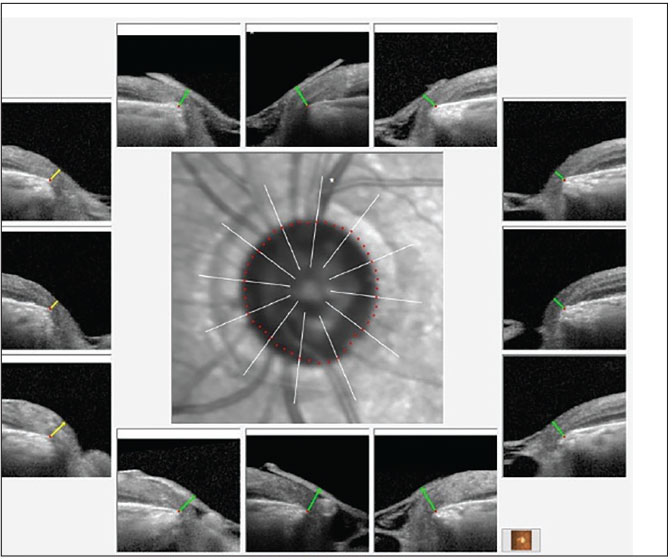
The posterior pole evaluations were consistent with the staging of glaucoma, with a 0.9x0.95 cup-to-disc ratio in the right eye and 0.65x0.75 in the left. The right neuroretinal rim was thin, especially temporally, with inferior temporal erosion of the rim. The left neuroretinal rim was thin, too, but the remaining tissue was plush and well perfused. These clinical findings were supported by OCT imaging, as seen in Figures 1 and 2. The physical examination of both maculae were consistent with only age-related retinal pigment epithelium granulation. The retinal vasculature was characterized only by mild arteriosclerotic retinopathy OU and a diffuse epiretinal membrane (mild) was present OD. There was no evidence of diabetic retinopathy and the peripheral retinal examination was essentially unremarkable.
 |
|
Fig. 2. BMO-MRW overview of the left neuroretinal rim. Note the overall thin neuroretinal rim, but with the remaining rim still adequate for relatively good visual field levels. Click image to enlarge. |
At the initial visit, I did not have previous records to review, but his complaint of decreased vision in the right eye was likely attributable to field loss associated with the advanced state of glaucoma. I asked him to continue his medication therapy and see me back in about six weeks so that I could better assess his visual needs, as well as to obtain a baseline visual field study. I was also hoping to receive his previous records in the interim so that I would have some comparative data to evaluate.
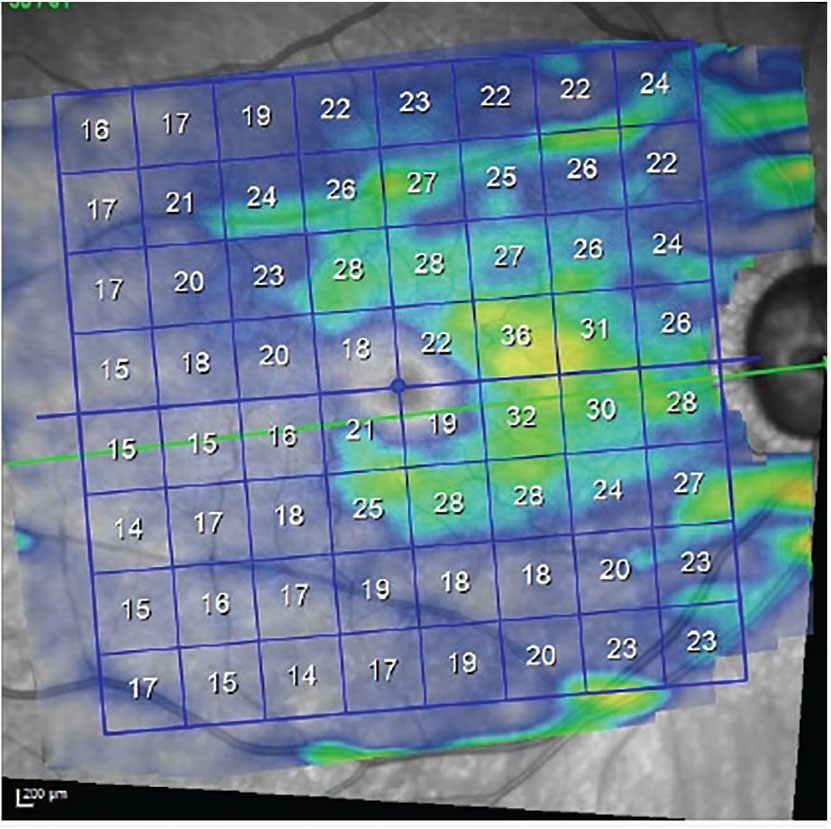
The patient presented as requested. Threshold visual fields in the right not surprisingly demonstrated a dense superior arcuate scotoma involving fixation, along with a dense inferior arcuate defect with a nasal step, also involving fixation. Field studies of the left demonstrated mild arcuate defects consistent with the level of structural damage seen. Gonioscopic examination of both anterior chamber angles demonstrated wide open angles or moderate trabecular pigmentation, and the presence of two iStent inject MIGS devices bilaterally. Refraction prior to gonioscopy did not improve visual acuities beyond his entering acuities. In Figures 3 and 4, we can see the effect of the glaucomatous damage on the macular ganglion cell layer in each eye, with the right suffering significantly more ganglion cell loss than the left.
I discussed with the patient that I did not feel that changing his spectacle Rx would offer any improvement in his vision, as the decreased vision was related to visual loss from glaucoma—not due to a refractive change. He disappointedly said that is essentially what his previous doctor had told him. I tried to be positive and reinforce that although we could not improve his vision, we would do everything possible to preserve his remaining vision. Similar notations were found in his previous provider’s records.
Spring the clock ahead to December 2023. Both structurally and functionally, the patient has remained stable OU. But on just about every visit, the patient complained about his vision in the right eye “not being good” and asked if we could make it somewhat better. Each time, trying a different tack, he was told that the vision in the right eye could not substantively be improved. In fact, in that two-year interim he presented urgently two times with complaints of not seeing well with the right eye, with each visit demonstrating the same structural and functional defects.
 |
|
Fig. 3. Ganglion cell thickness OD demonstrates loss of ganglion cells consistent with the visual field loss present in this eye, which involves fixation. Click image to enlarge. |
Earlier last week (as of this writing), he called the office asking for a referral to someone for a “YAG laser procedure” for his right eye. My technician fielded the call, gathering that he had spoken with an acquaintance who told him that after cataract surgery he too had poor vision, which improved after a YAG procedure, so he should get one, too. My tech reminded me he had already undergone a YAG capsulotomy in the right eye, with review of my records revealing no partial fibrosis nor capsular strands on the visual axis. I asked my tech to tell him that he already had that procedure done and that it would not help improve his vision. He called back later that same day and told us he scheduled an appointment himself. After discovering who he had made the appointment with, I spoke with that provider to explain the situation as to what they would be seeing upon his arrival.
Discussion
While I’m not worried about an unnecessary YAG procedure, these types of events do consume time. As it turns out, the patient was beginning to develop early dementia, as reported by his wife during a different phone conversation, which would certainly explain the repeated discussions about the decreased vision OD, but might also explain his fixation on improving vision in that eye. Be that as it may, what would normally be a straightforward case of glaucoma and normal chair time rather easily expanded into several calls that, in a perfect world, would not have been necessary.
But this is the practice of medicine and optometry, and it crosses all types of ophthalmic conditions from the anterior segment to the retina, optic nerve and other organ systems. Practicing good, ethical care involves clinical knowledge, empathy and communication, but it also involves an efficient office. Situations like these can eat up some of that efficiency.
Interestingly, just before I sat down to pen this column, my good friend Dr. Tom Cheezum sent me a note about a change in CPT coding for 2024. Tom is a coding guru, and when he saw this change he thought of me, as I see complex cases all day, most of which involve a significant amount of coordination of care. Apparently, as of January 1, primary care providers (such as ourselves), when seeing patients with chronic illnesses like glaucoma, can code G2211 as an add-on code to E/M services. As I gather, this code was introduced to help defray the costs associated with coordinating care, such as in cases like this one. While the reimbursement is not large (less than $20), for those of us seeing 30 or so chronic cases each day, this will help offset the extra time spent on these patients outside of the exam room.
In any event, if you offer good, compassionate care, you will be rewarded in one way or another. Besides, that’s our job anyway.
Dr. Fanelli is in private practice in North Carolina and is the founder and director of the Cape Fear Eye Institute in Wilmington, NC. He is chairman of the EyeSki Optometric Conference and the CE in Italy/Europe Conference. He is an adjunct faculty member of PCO, Western U and UAB School of Optometry. He is on advisory boards for Heidelberg Engineering and Glaukos.

