 |
| Basic AI algorithms may be used to advance prognosis of early diabetic retinopathy. Photo: Carolyn Majcher, OD. Click image to enlarge. |
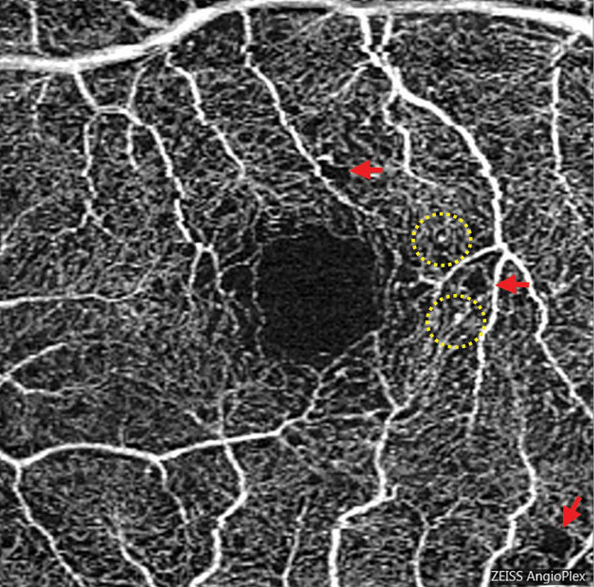
Early retinal disease progression with diabetes is characterized by microaneurysm presence. However, since capillary loss can occur before this presentation and long after, early assessment of the disease should encompass more than perfused vascular features, including microaneurysm number and neovascularization.
In a new study published in JAMA Ophthalmology, researchers evaluated advances in OCT angiography (OCT-A) using an AI algorithm for identifying diabetic macular ischemia (DMI) to assess and provide prognostic value on diabetic retinopathy progression, diabetic macular edema development and visual acuity deterioration among a cohort of diabetic patients.
DMI assessment was performed by a deep learning algorithm of superficial capillary plexus and deep capillary plexus OCT-A images. DMI presence was defined as “images exhibiting disruption of foveal avascular zone with or without additional areas of capillary loss,” and DMI absence was defined as “images presented with intact foveal avascular zone outline and normal distribution of vasculature.”
Diabetic patients were followed for at least four years, and models to evaluate the association between DMI and retinopathy progression, diabetic macular edema development and visual acuity deterioration were used.
Of the 321 eyes from 178 patients (47.8% female), 32.7% (n=105) displayed retinopathy progression, 10.3% (n=33) developed macular edema and 21.2% (n=68) experienced visual acuity deterioration over a median follow-up of 50 months.
After adjusting for factors of age, diabetes duration, fasting glucose, glycated hemoglobin, mean arterial blood pressure, retinopathy severity, ganglion cell-inner plexiform layer thickness, axial length and smoking, superficial capillary plexus/DMI and deep capillary plexus/DMI were associated with retinopathy progression and visual acuity deterioration.
The authors pointed to several reasons why the presence of deep capillary plexus/DMI improved the discriminative performance for edema development. One possibility is that deep plexus ischemia breaks down the blood-retina barrier and facilitates pericyte loss, both hallmarks of macular edema. Another may be that deep plexus ischemia causes dysfunction of Müller cells and reduces fluid absorption, leading to intraretinal fluid accumulation in the retina. Finally, since the deep capillary plexus is more closely connected to the venous channel anatomically, significant damage to the structure could affect venular drainage through outflow, making retinal fluid accumulation worse.
Visual acuity deterioration may be caused by reduced retinal sensitivity and prolonged electroretinogram wave, as previously reported. Involvement of DMI in different plexuses may explain varying phenotypes, like how the superior plexus may be accompanied by disorganized retinal inner layers, while the deep plexus is likely closely related to photoreceptor disturbance.
The authors concluded, “Our current results suggest that the presence of DMI in both plexuses at baseline fields the highest discriminative performance for predicting retinopathy progression. Our findings might help practitioners pay close attention to those presenting with DMI, especially to those with DMI in both superficial and deep capillary plexuses, as it potentially provides additional risk assessment for retinopathy progression.”1
Commentary accompanying the research paper, also published in JAMA Ophthalmology, provides more insight as to why these findings are important to consider, pointing to three main points.
For one, the research provides a foundation that may necessitate revisiting early diabetic retinopathy classification. That is, the staging system should include measures of subclinical impairment in capillary perfusion.
Second, the team makes a case for OCT-A adoption as standard of care when assessing early ischemic retinal disease in diabetic patients.
Lastly, the commentary highlights the importance of combining a fairly basic machine learning method with highly sensitive, noninvasive imaging markers to provide clinically significant prognostic information, in this case, about diabetic retinopathy.
The commentary authors note that “much like the observation that OCT and antivascular endothelial growth factor therapies have made focal laser photocoagulation essentially obsolete, we are standing at the edge of a new era in diagnosis and treatment of retinal vascular disease enabled by the combination of OCT-A and AI.”2
1. Yang D, Tang Z, Ran A, et al. Assessment of parafoveal diabetic macular ischemia on optical coherence tomography angiography images to predict diabetic retinal disease progression and visual acuity deterioration. JAMA Ophthalmol. May 25, 2023. [Epub ahead of print]. 2. Kashani AH, Liu A, Jones C. Optical coherence tomography angiography, artificial intelligence, and the missing capillaries. JAMA Ophthalmol. May 25, 2023. [Epub ahead of print]. |


