 |
|
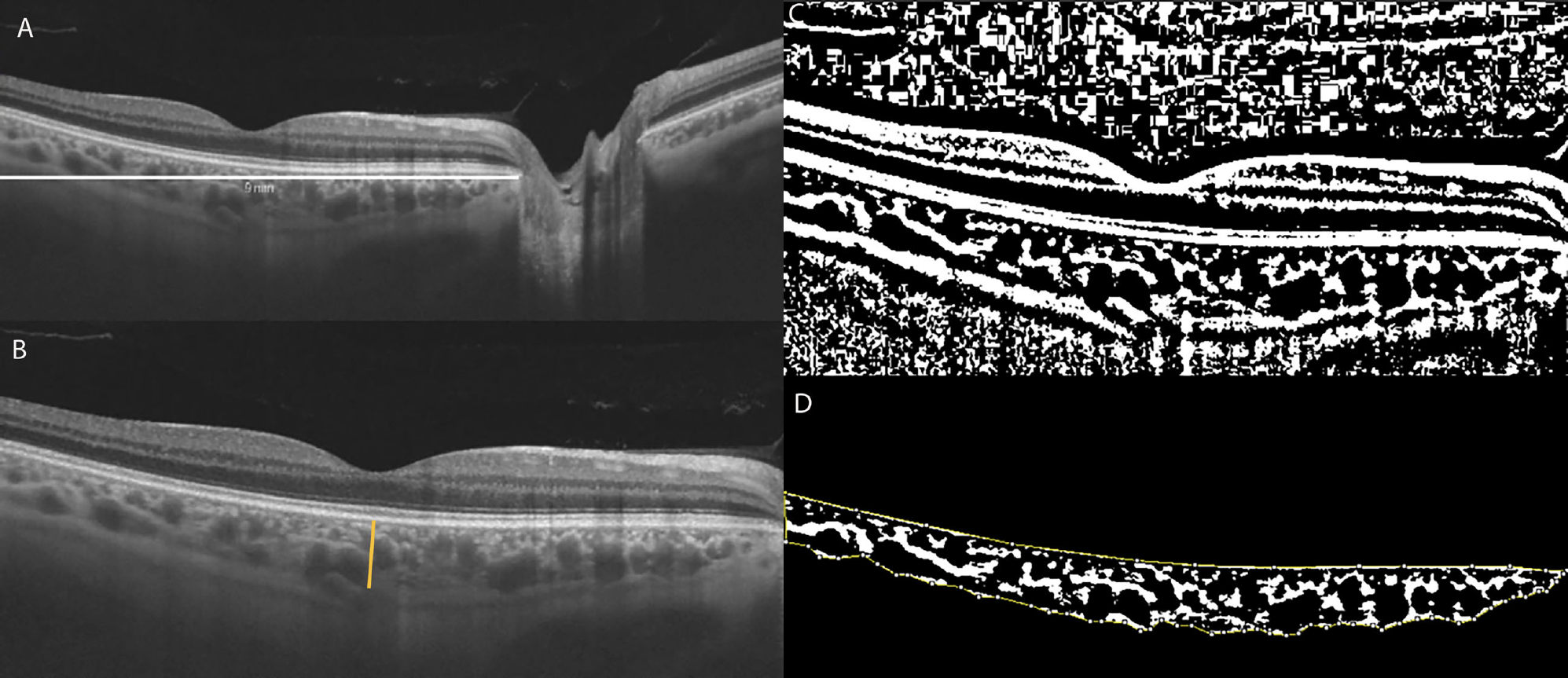
CVI is calculated by demarcating a 9mm horizontal B-scan at the fovea (fig. A, white line) and the subfoveal choroidal thickness (fig. B, orange line). The image is then binarized (fig. C), with the dark pixels representing the vessels’ luminal area and the white pixels the stromal area. Finally, the total choroidal area—the space between the yellow lines—is isolated with the RPE representing the anterior boundary and the scleral-choroidal interface as the posterior boundary, across the entire length of the scan. Total luminal area divided by total choroidal area represents the CVI, a measure of the structure’s vascular capacity. The study in question found a lower CVI in DR patients, indicating that low perfusion and proliferative changes are the major pathological effects in the choroid. Photo: Mucciolo DR, et al. Front. Ophthalmol. 2021; https://doi.org/10.3389/fopht.2021.755058. Click image to enlarge. |
Diabetic retinopathy (DR) is one of the major causes of blindness among working-aged adults worldwide, but its pathophysiology remains somewhat unclear. In a recent study, researchers aimed to evaluate the differences in the subfoveal choroidal thickness and choroidal vascularity index (CVI) using OCT of diabetes patients with and without retinopathy. CVI is a newer metric derived from OCT data that quantifies the vascular area of the choroid as a percentage of the entire structure. Higher values indicate greater vascular capacity.
A total of 23 cross-sectional studies comprising 2,534 eyes (1,464 with DR, 1,070 without) were included in the systematic review and meta-analysis.
The study showed that the CVI was decreased and the subfoveal region was thicker in DR patients after adjusting for axial length. It further showed that the CVI was significantly decreased in mild to moderate nonproliferative DR (NPDR) and the subfovel choroidal tissue was significantly thicker in severe cases of both nonproliferative and proliferative DR.
In the subgroup analysis adjusted for axial length, the team demonstrated that SFCT was significantly increased in DR eyes which was different from the main analysis indicating a potential influence of axial length on SFCT.
Previous studies showed a significant decrease in subfoveal choroidal thickness and CVI in diabetes patients compared with healthy controls, while this study showed a significant decrease in CVI in mild to moderate NPDR compared with non-DR, indicating that the ischemic change and reduced blood flow in the choroidal vasculature continues to progress in early stages of DR.
“We hypothesized that choroidal neovascularization caused by elevated VEGF may explain the increase in [subfoveal choroidal thickness) in later stages of DR,” the authors noted. “Choroidal neovascularization contributes to the stromal component while choroidal vasodilation increases the luminal component. Due to the decrease in CVI, we consider the increase is mainly in the stromal component. In short, choroidal vascular constriction and low blood flow reduce the luminal component of the choroid and thereby decrease CVI, whereas proliferative changes in later DR stages increase the stromal component,” resulting in an increased thickness.
To explore the heterogeneity across different OCT instruments for the measurement of CVI, the authors conducted a subgroup analysis according to device type. Findings derived from SD-OCT corresponded to the main analysis; however, those calculated with SS-OCT showed a different result, possibly due to the relatively small number of included studies (just three), the authors explained.
Choroidal vascularity index and subfoveal choroidal thickness “might be valuable parameters for discriminating DR from non-DR,” the authors concluded. “This facilitates a better understanding of the role of the choroid in the pathophysiology of DR and provides references for ophthalmologists to detect the onset of DR in diabetes mellitus patients.”
Jiang J, Liu J, Yang J, Jiang B. Optical coherence tomography evaluation of choroidal structure changes in diabetic retinopathy patients: a systematic review and meta-analysis. Front Med. October 20, 2022. [Epub ahead of print]. |


