In recent years, the eyecare community has done an admirable job of raising awareness to the dangers wrought by myopia and reframing the condition as a disease amenable to intervention. Once passed off as an inconvenience to be remedied with glasses, myopia poses ocular health risks that may not manifest for decades. Patient-centered care of highly myopic individuals relies on understanding the risk of development of this ocular pathology as well as knowing the long-term potential complications related to any past procedures. Let’s dive into the vulnerabilities related to high myopia through addressing chronic pathology, with a focus on patient-driven concerns related to long-term prognosis.
While local and global projections related to myopia, high-myopia and associated visual impairment hold steady in their expected impact on visual function and ocular health, they reflect the necessary role of managing providers for prevention, early intervention, continued care and education that begins in the exam room.1,2 Changes in pediatric myopia management and addressing early pediatric myopia clinical ideas are meaningful in altering the potential course of the disease, associated pathology and visual dysfunction in adulthood. However, we still must be ready to meet the needs of our growing population of highly myopic adult patients.
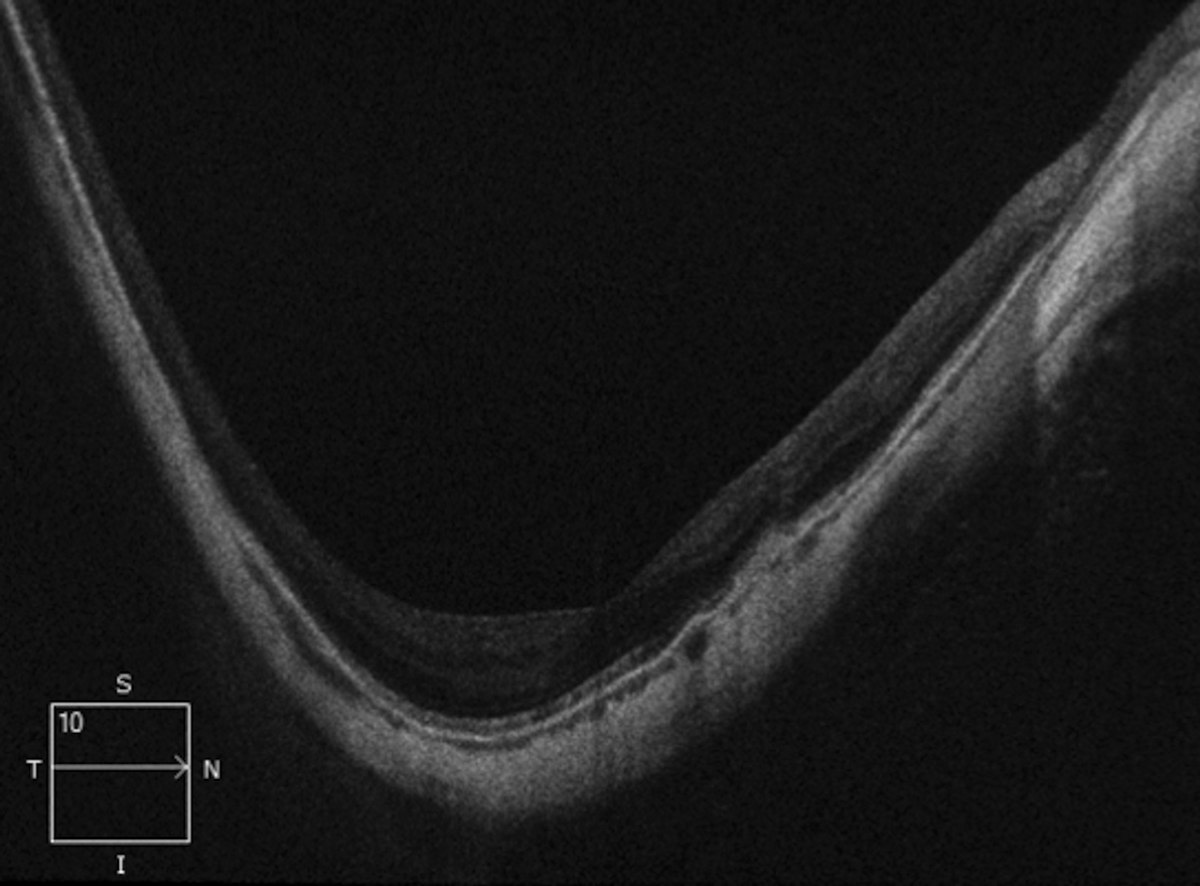
 |
|
Macular posterior staphyloma with severe choroidal thinning and RPE disruption on spectral domain OCT. Click image to enlarge. |
What’s in a Name?
“Degenerative” myopia, “pathologic” myopia, “malignant” myopia or just “myopia”? Difficulty in labeling of this condition often stems from the variability in literature and absence of a comprehensive, globally accepted definition of high myopia and its associated pathologies. In speaking about high myopia, most commonly we refer to refractive error (spherical equivalent) of -6.00D or higher or axial length at least 26.0mm or 26.5mm; however, there is no clear scientific rationale for how these threshold values were determined.3-5 Importantly, high myopia does not imply pathologic myopia. The latter is an umbrella term that includes a host of clinical diagnoses that may affect the optic nerve, neurosensory retina, Bruch’s membrane, choroid and sclera, which are specifically related to posterior staphyloma formation and variability in three-dimensional globe-shape.3-6
Back to Square One
Factors leading to myopia development are complex, with a basis in genetics and significant environmental impact on its development and progression.1,7-10 While the physiological mechanisms behind myopia development are still being investigated, animal models have demonstrated that, in response to retinal defocus, a signaling cascade beginning in the neurosensory retina leads to biochemical changes in the retinal pigment epithelium (RPE), choroid and sclera that result in collagen remodeling and increased axial length.7 Near work activities, light levels and time outdoors have been proposed to contribute to myopia development and progression, and epidemiological research continues to explore additional environmental factors that may be implicated.7 Data from twin studies have reported high heritability (90%) of myopia; however, a recent meta-analysis estimated the heritability of myopia to be only 18.4%.8,9
Based on that, the ability to predict the development of myopia based on genetics alone falls half-way between chance and perfect prediction (area under the curve = 0.75).9 Early and regular comprehensive eye examinations, including cycloplegic refraction in line with clinical practice guidelines, are more practical to identify those who are progressing towards myopia, or those who have myopia, in order to employ meaningful early intervention with the goal of slowing future progression than genetics alone.11
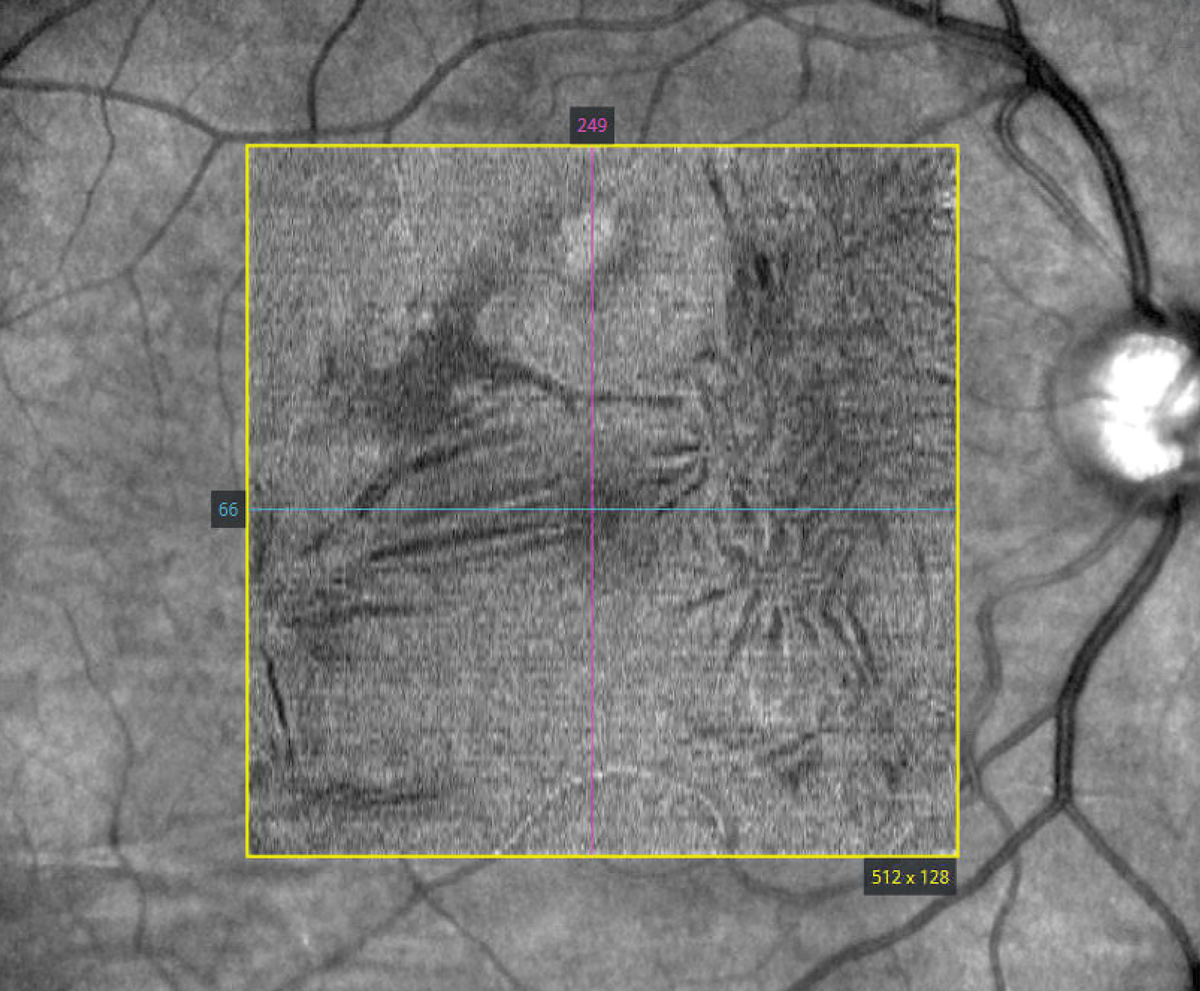 |
|
Epiretinal membrane following barrier laser photocoagulation of retinal break en face. Click image to enlarge. |
Sight-threatening Sequelae
Vision loss due to pathology associated with high myopia is a substantial source of global visual impairment.2 With a 6mm increase in axial length (24mm to 30mm), the distance between the optic disc and ora serrata has been described to increase by 4.3mm.12 Being aware of this sets the stage for a deeper understanding of the wide variety of potential peripheral retinal, macular and optic nerve pathology that encompasses pathologic myopia. While peripheral retinal pathologies leading to retinal tear or retinal detachment are significant complications associated with high myopia, here we focus on the chronic ocular conditions that have a high disease burden due to chronic progressive vision loss.
Cataract Development and Treatment
Visually significant nuclear sclerosis occurs at a younger age in high myopes, and myopic individuals have been shown to have an increased likelihood of developing posterior subcapsular cataract.4,12,13 Meeting the high visual expectations of younger, highly myopic individuals who undergo cataract surgery often involves a goal of contact lens or spectacle independence; however, for those with the macular pathology or axial length greater than 28mm, the use of multifocal intraocular lenses is controversial.14,15 High myopes are more likely to experience intraocular lens tilt and intraocular lens decentration inducing defocus, as well as coma-like aberration (possibly due to larger capsular bag in myopic eyes), compared with standardized diameters of intraocular lenses or zonular weakness.14 Following cataract surgery, high myopia has also been determined to be an additional risk factor for the development of visually significant posterior capsular opacification.16
Retinal tear and retinal detachment are established postsurgical complications with an increased risk amongst high myopes (axial length > 26mm) and those with lattice degeneration.17 Retinal tear or detachment is most likely to occur within the first six months following cataract surgery, with a median time of approximately 4.5 months following the procedure.17 Identifying highly myopic patients who have additional risk of specific postoperative complications, along with patient education and a plan set with office triage staff to best manage emergency concerns when they arise, can lead to early detection and referral for treatment.
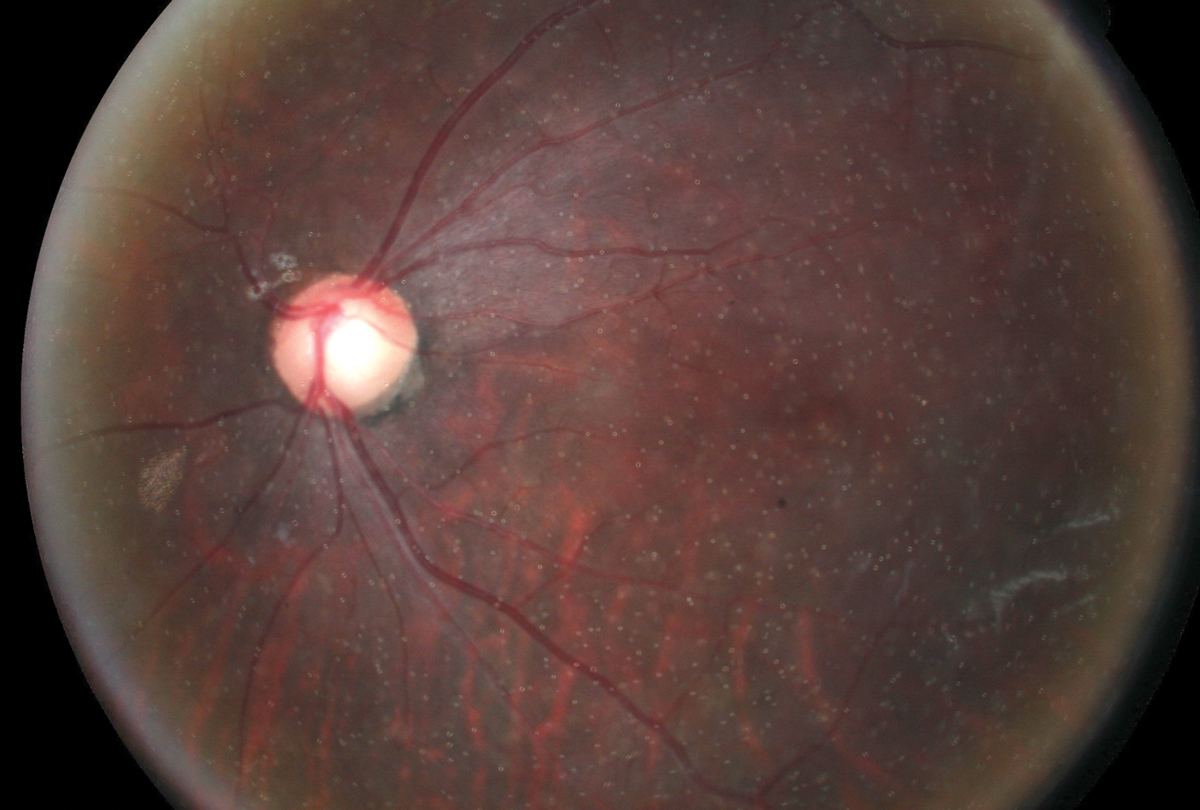
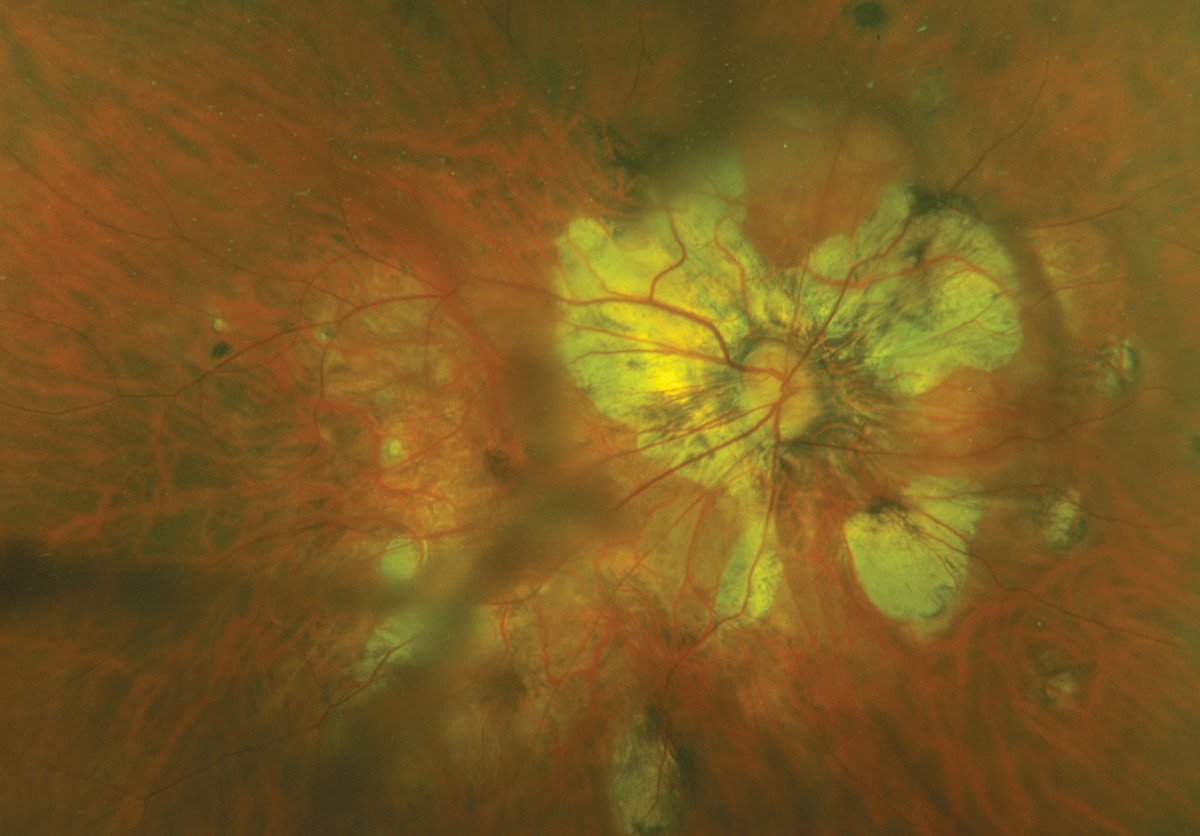 |
| Patchy chorioretinal atrophy in a high myope with posterior staphyloma. Click image to enlarge. |
Myopic Maculopathy
This term is a general descriptor for the neovascular, atrophic and tractional conditions that affect the macula in high myopes. While classification systems have been developed with the goal of consistent description and standardization among clinical trials and epidemiological studies, they are not commonly used in a clinical environment.3,5,18
Posterior staphyloma, a scleral outpouching with increased curvature, is an important feature of pathologic myopia and a primary feature that leads to macular pathology. Posterior staphyloma may occur in nonmyopic eyes, and therefore does not necessarily rule in the diagnosis of pathologic myopia.5,10 As axial length increases, the retina, choroid and sclera become thin, which may lead to foveoschisis, myopic macular atrophy, macular neovascularization or serous retinal detachment associated with dome-shaped macula.4,5,10
Vitreous liquefaction and posterior vitreous detachment occur in younger myopic eyes, and the development of epiretinal membrane is common and found in up to 45.7% of highly myopic individuals.5,19 Myopic tractional maculopathy, which includes vitreomacular traction, foveoschisis and macular hole, is primarily due to the presence of posterior staphyloma and the inability of the retina to match the curvature of the more curved underlying sclera.5,10 Generally, myopic tractional maculopathy progresses slowly. Observation is recommended until best-corrected visual acuity declines in line with anatomic worsening determined by OCT, where surgery will be considered.5,10
Chorioretinal degeneration leading to atrophic myopic maculopathy is a visually devastating end-stage disease following development and treatment of macular neovascularization. It can be a primary outcome of myopic choroidopathy with similar visual consequences to geographic atrophy occurring in age-related macular degeneration, despite having a different underlying pathogenesis.5,10 Myopic chorioretinal degeneration begins in the posterior pole as patchy areas of RPE and choroid loss, often with RPE hyperplasia at the edge of the lesion, and increases over time to form large atrophic lesions. In addition to fundus images for documentation, OCT and fundus autofluorescence are valuable to monitor progression of atrophy.4,5,10,20,21 While recent therapeutic advancements for the treatment of geographic atrophy secondary to age-related macular degeneration are available to slow disease progression, no therapeutic strategies are currently approved for the treatment of myopic macular atrophy.
 |
| Segmentation error and imaging acquisition error due to parapapillary staphyloma. Click image to enlarge. |
The development of macular choroidal neovascularization is associated with the presence of lacquer cracks, or linear defect of the RPE-Bruch’s membrane complex in high myopes.4,5,10 Macular neovascularization in high myopia tends to be highly responsive to anti-VEGF therapy when treated promptly.5,10
Glaucoma Risk
Our understanding of the risk factors related to the development of primary open-angle glaucoma continues to evolve. Recently published 10-year follow up data of the Beijing Eye Study identified high myopia (≤-6.00D) to be associated with a 7.3-fold increased risk for the development of open-angle glaucoma compared with emmetropic eyes.22 As myopia increases, the risk of open-angle glaucoma increases, with a meta-analysis describing a 20% increase in the risk of development of glaucoma with every one diopter increase in myopia.23 The risk profile increases with spherical equivalent higher than -6.00D and increases further from -8.00D.23
The presumed reason for increased risk of glaucoma in myopic individuals has been related to a thinner, stretched sclera and reduced scleral rigidity compared with emmetropic eyes.22,24 The lamina cribrosa also stretches and thins with increased axial elongation, increasing the shearing stress on the pores of the lamina cribrosa and the axons that pass through them.25 Ischemic stress to the lamina cribrosa may be increased in highly myopic eyes due to an increase in the distance between the lamina and its vascular supply.25 Increased oxidative stress driving cell death as a result of RPE and choroidal atrophy in highly myopic eyes has also been proposed to be a potential contributing mechanism to increased glaucoma risk in highly myopic eyes.26
Nonglaucomatous optic neuropathy due to high myopia may be challenging to differentiate from open-angle glaucoma.25 Axial elongation and increased temporal parapapillary atrophy can cause physical stretching of ganglion cell axons between the fovea and optic disc, resulting in parafoveal visual field defect in the absence of glaucomatous optic disc appearance.25,27 The prevalence of nonglaucomatous optic neuropathy due to high myopia was recently described to be 29.3% in a Russian population of individuals over the age of 40 with increased likelihood of optic neuropathy with increased axial length.27
Long-Term
After surgical management of a retinal tear or detachment, it may be difficult to distinguish the subtleties between expected findings associated with treatment and those that may prompt re-referral. The purpose of barrier laser photocoagulation in retinal tear or detachment treatment is to create adherence between the retina and choroid to prevent liquefied vitreous from entering the subretinal space.28 Considering the induced traction caused by photocoagulation at the boundary of detached and attached retina, patients who are critical observers may continue to have symptoms of localized photopsia in the treatment area.
Epiretinal membrane (ERM) formation following laser photocoagulation or pars plana vitrectomy may also occur. While some ERMs may develop and progress quickly, asymptomatic or minimally symptomatic development following retinal laser may also occur.28 This typically happens in younger myopic patients who have undergone pars plana vitrectomy, which may present as a form of form-fruste proliferative vitreoretinopathy. Asymptomatic or the minimally symptomatic development of epiretinal membrane following retinal laser may also occur. If a patient becomes visually symptomatic, re-referral for surgical evaluation is warranted.28
Tamponade agents are surgical adjuncts used in retinal detachment repair procedures that encourage chorioretinal adherence. While gas tamponade (i.e., SF3 or C3F8) is preferred from a patient perspective due to its ability to resorb, long-term silicone oil tamponade may be used in cases of complex retinal detachment repair, including cases with history of proliferative vitreoretinopathy, ocular trauma or severe diabetic retinopathy.29 While silicone oil is generally removed three to six months after insertion at the discretion of the operating surgeon, it may be left indefinitely, depending on patient risk factors.29 Silicone oil emulsification generally begins within the first year following injection. While emulsification alone is not a reason for removal, it may lead to complications including corneal endothelial dysfunction and corneal decompensation, intraocular inflammation and open-angle glaucoma.29,30
Clinically, early emulsification is observed as small bubbles in the vitreous cavity. Silicone oil can migrate into the anterior chamber and result in “reverse hypopyon” with emulsified silicone oil particles, which are lighter than the aqueous, settling superiorly in the anterior chamber, which is best observed with gonioscopy in early cases.30 Silicone oil present in the anterior chamber can result in corneal endothelial cell loss and may enter and clog the trabecular meshwork.30
The resulting reduction in aqueous outflow can lead to elevated intraocular pressure (IOP), which has been described to occur in as many as 56% of eyes, and open-angle glaucoma.29,31 Any patient with silicone oil in the vitreous cavity should remain under the care of a retinal surgeon, in addition to a comanging provider’s care for periodic evaluation for known potential ocular complications, including elevated IOP that may require IOP-lowering therapy or necessitate oil removal.
 |
|
Silicone oil emulsification following pars plana vitrectomy for treatment of rhegmatogenous retinal detachment. Click image to enlarge. |
High Myopia and Imaging
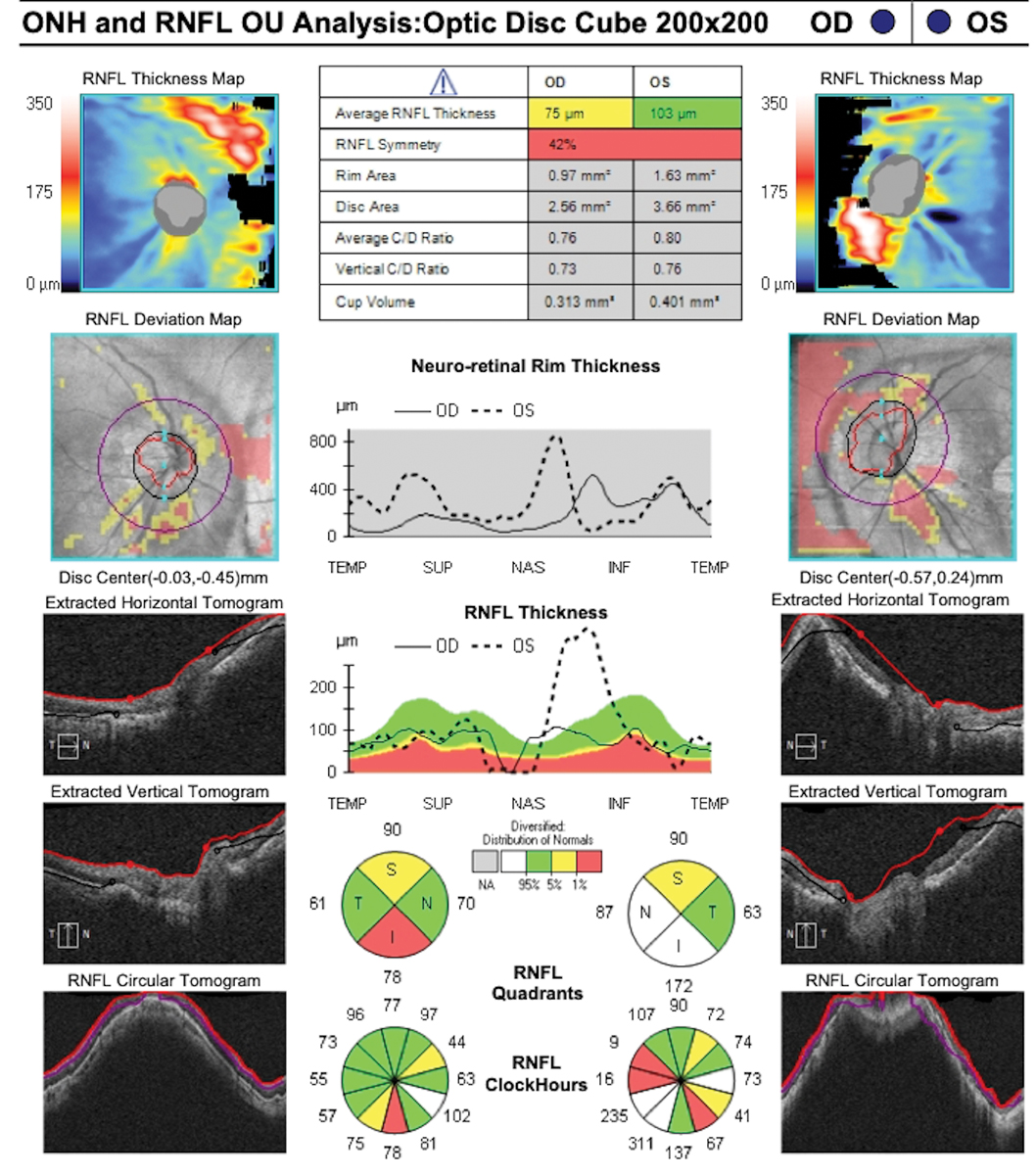
OCT is an important tool for structural evaluation of the optic nerve and macula, which, due to the increased curvature of a posterior staphyloma, can make imaging retinal pathology challenging. Reference databases comparisons of nerve fiber layer thickness, optic disc parameters and ganglion-cell or ganglion-cell complex parameters of commercially available OCT systems in the United States have traditionally excluded eyes with refractive error greater than -6.00D. Reference database parameters should be made with a reflection of the individual, their ocular feature and whether they may or may not be represented within the reference database.
With increased axial length, transverse magnification of the imaging area of the OCT is reduced, which results in a larger retinal area imaged compared with an eye of average axial length.32 This may lead to an underestimation of peripheral macular thickness due to inclusion of additional retinal area being imaged in a high axial length eye, which would otherwise not be included in an eye of average axial length, or an underestimated of RNFL thickness due to the lower density of axons further from the optic disc imaged by the induced larger scan area.32 OCT-based optic disc parameters, including neuroretinal rim thickness, rely on accurate determination of Bruch’s membrane opening, which may not be discernible in highly myopic eyes, especially in the presence of significant parapapillary atrophy impacting reproducibility and software-based segmentation.33\
Takeaways
Strategies employed during childhood aimed to delay, reduce or prevent the development high myopia are key to disrupting the rapidly rising trend of this condition and its associated ocular pathology. An understanding of the chronic ocular pathology associated with increased axial length and its early identification, along with individualized monitoring schedules, retinal surgical referral when indicated and comanagement with other providers, is central for maximizing long-term visual outcomes and the unique needs of highly myopic individuals.
Dr. Steen is an associate professor at Nova Southeastern University College of Optometry where she serves as director of the Glaucoma Service, coordinator of the Primary Care with Emphasis in Ocular Disease Residency and teaches courses in glaucoma and ocular pharmacology. Her financial disclosures include Bausch + Lomb, Santen, Iveric Bio, Alcon, Viatris, Ocuphire, Ocuterra, Allergan, Thea Pharma, Ocuphire and Carl Zeiss Meditec.
1. Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036-42. 2. Fricke TR, Jong M, Naidoo KS, et al. Global prevalence of visual impairment associated with myopic macular degeneration and temporal trends from 2000 through 2050: systematic review, meta-analysis and modelling. Br J Ophthalmol. 2018;102(7):855-62. 3. Flitcroft DI, He M, Jonas JB, et al. IMI - defining and classifying myopia: a proposed set of standards for clinical and epidemiologic studies. Invest Ophthalmol Vis Sci. 2019;60(3):M20-30. 4. Haarman AEG, Enthoven CA, Tideman JWL, et al. The complications of myopia: a review and meta-analysis. Invest Ophthalmol Vis Sci. 2020;61(4):49. 5. Ruiz-Medrano J, Montero JA, Flores-Moreno I, et al. Myopic maculopathy: current status and proposal for a new classification and grading system (ATN). Prog Retin Eye Res. 2019;69:80-115. 6. Moriyama M, Ohno-Matsui K, Modegi T, et al. Quantitative analyses of high-resolution 3D MR images of highly myopic eyes to determine their shapes. Invest Ophthalmol Vis Sci 2012;53(8):4510-8. 7. Chakraborty R, Read SA, Vincent SJ. Understanding myopia: pathogenesis and mechanisms. In: Ang M, Wong T, eds. Updates on Myopia. Singapore, Springer, 2020. 8. Hammond CJ, Snieder H, Gilbert CE, Spector TD. Genes and environment in refractive error: the twin eye study. Invest Ophthalmol Vis Sci. 2001;42(6):1232-6. 9. Hysi PG, Choquet H, Khawaja AP, et al. Meta-analysis of 542,934 subjects of European ancestry identifies new genes and mechanisms predisposing to refractive error and myopia. Nat Genet. 2020;52(4):401-7. 10. Ohno-Matsui K, Wu PC, Yamashiro K, et al. IMI pathologic myopia. Invest Ophthalmol Vis Sci. 2021;62(5):5. 11. American Optometric Association. Evidence-Based Clinical Practice Guideline: comprehensive pediatric eye and vision examination aoa comprehensive pediatric and vision examination. 2017. www.aoa.org/aoa/documents/practice management/clinical guidelines/ebo guidelines/comprehensive pediatric eye and vision exam.pdf. Accessed August 31, 2023. 12. Panda-Jonas S, Auffarth GU, Jonas JB, Jonas RA. Elongation of the retina and ciliary body in dependence of the sagittal eye diameter. Invest Ophthalmol Vis Sci. 2022;63(10):18. 13. Pan CW, Cheng CY, Saw SM, et al. Myopia and age-related cataract: a systematic review and meta-analysis. Am J Ophthalmol. 2013;156(5):1021-33.e1. 14. Wang L, Jin G, Zhang J, et al. Clinically significant intraocular lens decentration and tilt in highly myopic eyes: a swept-source optical coherence tomography study. Am J Ophthalmol. 2022;235:46-55. 15. Grzybowski A, Kanclerz P, Tuuminen R. Multifocal intraocular lenses and retinal diseases. Graefes Arch Clin Exp Ophthalmol. 2020;258(4):805-13. 16. Donachie PHJ, Barnes BL, Olaitan M, et al. The Royal College of Ophthalmologists’ National Ophthalmology Database study of cataract surgery: report 9, risk factors for posterior capsule opacification. Eye (Lond). 2023;37(8):1633-9. 17. Morano MJ, Khan MA, Zhang Q, et al. Incidence and risk factors for retinal detachment and retinal tear after cataract surgery: IRIS Registry (Intelligent Research in Sight) analysis. Ophthalmol Sci. 2023;3(4):100314. 18. Ohno-Matsui K, Kawasaki R, Jonas JB, et al. International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol. 2015;159(5):877-83.e7. 19. Ripandelli G, Rossi T, Scarinci F, et al. Macular vitreoretinal interface abnormalities in highly myopic eyes with posterior staphyloma: five-year follow-up. Retina. 2012;32(8):1531-8. 20. Ang M, Wong CW, Hoang QV, et al. Imaging in myopia: potential biomarkers, current challenges and future developments. Br J Ophthalmol. 2019;103(6):855-62. 21. Itoi M, Hieda O, Kusada N, et al. Progression of myopic maculopathy: a systematic review and meta-analysis. Eye Contact Lens. 2023;49(2):83-7. 22. Wang YX, Yang H, Wei CC, et al. High myopia as risk factor for the 10-year incidence of open-angle glaucoma in the Beijing Eye Study. Br J Ophthalmol. 2023;107(7):935-40. 23. Ha A, Kim CY, Shim SR, et al. Degree of myopia and glaucoma risk: a dose-response meta-analysis. Am J Ophthalmol. 2022;236:107-19. 24. Sergienko NM, Shargorogska I. The scleral rigidity of eyes with different refractions. Graefes Arch Clin Exp Ophthalmol. 2012;250(7):1009-12. 25. Jonas JB, Wang YX, Dong L, Panda-Jonas S. High myopia and glaucoma-like optic neuropathy. Asia Pac J Ophthalmol (Phila). 2020;9(3):234-8. 26. Francisco BM, Salvador M, Amparo N. Oxidative stress in myopia. Oxid Med Cell Longev. 2015;2015:750637. 27. Bikbov MM, Iakupova EM, Gilmanshin TR, et al. Prevalence and associations of nonglaucomatous optic nerve atrophy in high myopia: the Ural Eye and Medical Study. Ophthalmology. July 17, 2023. [Epub ahead of print]. 28. Israilevich R, Salabati M, Mahmoudzadeh R, et al. Secondary epiretinal membrane after laser retinopexy for retinal tear or localized retinal detachment: surgical outcomes and optical coherence tomography structural analysis. Retina. 2022;42(1):38-45. 29. Toklu Y, Cakmak HB, Ergun SB, et al. Time course of silicone oil emulsification. Retina. 2012;32(10):2039-44. 30. Shimmura-Tomita M, Takano H, Tanaka Y, et al. Status of corneal endothelial cells in the presence of silicone oil in the anterior chamber. Sci Rep. 2021;11(1):14055. 31. de Corral LR, Cohen SB, Peyman GA. Effect of intravitreal silicone oil on intraocular pressure. Ophthalmic Surg. 1987;18(6):446-9. 32. Niyazmand H, Lingham G, Sanfilippo PG, et al. The effect of transverse ocular magnification adjustment on macular thickness profile in different refractive errors in community-based adults. PLoS One. 2022;17(4):e0266909. 33. Zheng F, Wu Z, Leung CKS. Detection of Bruch’s membrane opening in healthy individuals and glaucoma patients with and without high myopia. Ophthalmology. 2018;125(10):1537-46. |

