In 1942, physician Theodore Terry made the connection between what he believed was a pathological condition of the embryonic hyaloid system and prematurity. He named the disease retrolental fibroplasia (RLF) but based this on the appearance of what we now know as stage 5 retinopathy of prematurity.1
Since then, other physicians have found an association between the disease and oxygen levels administered to premature infants in the hospital but determined that abnormalities of the hyaloid system were not present.2-4 As further understanding of the condition developed, RLF became known as retinopathy of prematurity (ROP).
ROP is a leading cause of vision loss in children, the National Eye Institute reports.5 In the United States alone, 14,000 to 16,000 premature low birth weight infants (less than 1,250g, or about 2.75lbs) will be affected by ROP annually and approximately 1,500 infants will develop ROP severe enough to require treatment.5 Despite available treatment, about 400 to 600 infants with ROP still become legally blind each year.5 Through improved advanced knowledge and medical technology, more infants are being born with low birth rates.
The good news: Research in the diagnosis and treatment of ROP has greatly enhanced our knowledge of the disease.6-8 Unfortunately, fewer eye care professionals are available and willing to monitor and treat infants who have ROP.9 Reasons for this decline include unfamiliarity of ROP and the complexity of scheduling visits to treat and monitor it. Medical liabilitypatients and families can currently sue until the child reaches the age of 19is another reason for the decline.
Even so, proper examination is vital for these infants to receive the appropriate care. Once the ROP has been treated or resolved, the child requires long-term monitoring for associated ocular conditions, including strabismus, amblyopia, myopia and retinal detachments. As eye-care professionals, we should be willing to examine these patients when needed through both monitoring examinations and long-term follow-up for the ocular effects of the disease.
This article describes the necessary examination, treatment and follow-up.
Normal Retinal Development
Before we can define ROP we must understand the normal development of the retinal vasculature, which begins during the fourth month of pregnancy and reaches completion sometime during the third trimester.10,11 Fetal vascular development proceeds from the optic nerve to the peripheral retina. Vascularization progresses rapidly in the sixth month of gestation as capillaries develop into arteries and veins.
Some of the side branches retract and atrophy, forming capillary-free perivascular zones that are dependent on oxygen concentration of the blood. An increase in the oxygen levels widens the capillary-free zone, while a decrease in the oxygen levels increases various angiogenic factors, including platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF).12
During the seventh month of gestation, the vessels form an advancing line of vasculature into the peripheral retina; this is also the time at which most preterm infants are at highest risk of developing ROP.13,14 At this time, gap junctions are thought to constitute a barrier to the normal progression of retinal capillaries and may be the cause of proliferation of the peripheral retinal capillaries toward the vitreous observed in ROP.15
Constant pruning of the capillary bed continues with the ongoing development and dropout of new vessels, which correlates with reduced VEGF messenger RNA production and endothelial cell apoptosis.16 This is regulated by fluctuation in oxygen levels. The vessels become mature when they are surrounded by a supportive matrix and are no longer susceptible to pruning by hypoxia.17 Once the retina has been completely vascularized, there is no more risk for the development of ROP.
How ROP is Defined
The hallmark feature of ROP is a disruption of the normal vascularization process, tortuosity of posterior pole vessels and neovascular- ization leading to a retinal detachment. International classification of ROP originally occurred in 1984, was expanded in 1987, and revisited in 2005 due to advances in knowledge of the disease and improved technology.18 This international classification defines ROP by the location of the disease, the number of clock hours involved, staging of the disease, and the absence or presence of plus disease (more on this below).
The retina is divided into three zones, all centered on the optic nerve, to express the location of the disease (see The Three Zones of the Retina, below). Zone I is defined as a diameter twice the distance between the fovea and the center of the optic nerve. Clinically, this is approximately the area of the retina seen through a 28D lens when the view is centered on the optic nerve. Zone II is a circle that extends from the nasal ora serrata toward the temporal ora serrata. Zone III is a crescent encompassing the temporal area of the retina to the temporal ora serrata that is not included in zone II.
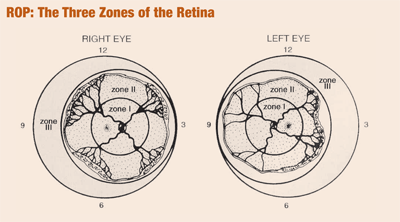
A coronal view depicting the zone borders and clock hours used to describe the location and extent of ROP.
The extent of the disease is defined in clock hours. Three oclock indicates the nasal aspect of the right eye and the temporal aspect of the left eye. Care is made to note consecutive and total clock hours involved when monitoring disease progression.
ROP is described by individual clock hours; however, staging for the entire eye is based on the most severe presentation of ROP that is seen by the examiner.18 The stages:
Stage 1. Normal avascularized retina appears to be grayish and opaque in color. A definite demarcation line, which is flat, between the vascularized and avascularized retina defines stage 1 ROP.
Stage 2. The transition into stage 2 ROP occurs when a ridge, with height and width, appears between the vascularized and avascularized areas.
Stage 3. ROP is classified as stage 3 when there is neovascularization along the ridge that extends into the vitreous.
Stages 4, 4A and 4B. When a partial retinal detachment occurs, the ROP has reached stage 4 ROP. If the fovea is not involved, it is termed stage 4A. If the fovea is off with a partial detachment, it is stage 4B.
Stage 5. At this stage, a complete retinal detachment has occurred.
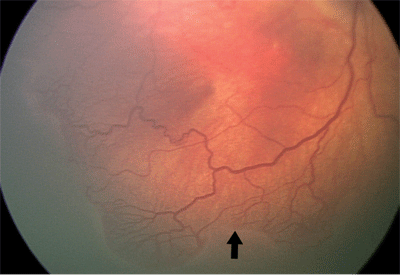
In Stage 1 retinopathy of prematurity, the normal avascularized retina appears to be grayish and opaque in color. A definite, flat, demarcation line between the vascularized and avascularized characterizes this stage.
Along with the changes at the vascular-avascular interface, vessel dilation and tortuosity of the major arteries and veins in the posterior pole can develop. Dilation and tortuosity that occurs in at least two of the four quadrants around the optic nerve is termed plus disease (see photo, below), and a + sign is added to the notation; for example, stage 3 ROP with plus disease is recorded as stage 3+ ROP.
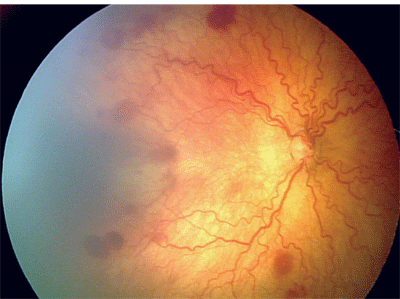 |
| Plus disease in ROP. Note that the dilation and tortuosity is in at least two quadrants around the optic nerve. |
Contributory Factors
While birth weight appears to have the largest impact on the potential for development of ROP, other environmental/biological factors likely contribute to its development.19 These include vascular endothelial growth factor (VEGF), oxygen, steroids and light.
VEGF is considered to play a crucial role in the neovascularization of many proliferative retinal diseases, including ROP. Increased levels of VEGF have been found in the vitreous of patients who have various proliferative retinopathies, and the vitreous fluid from these patients has been shown to stimulate endothelial cell growth in vitro.20 Expression of VEGF in utero is likely to be involved in the control of the normal development and regression of retinal vessels, while untimely changes in oxygen levels may cause dysregulation of VEGF, leading to inappropriate vaso-obliteration or exaggerated vaso-proliferation.
This concept is supported by clinical studies in which fluctuation in oxygen levels leads to more severe ROP than overall oxygen tension.21 Studies are currently underway to find a way to use anti-angiogenic agents to specifically target the pathological vessel development without interfering with normal angiogenesis.22-24
The relationship between oxygen levels and ROP has been researched since its discovery. Neonatal units closely monitor the level of supplemental oxygen exposure in preterm infants, although the proper balance has yet to be discovered. Early on, researchers suggested that supplemental oxygen would reduce the incidence of ROP by preventing the ischemic phase and down-regulating the angiogenic growth factors.25 However, the Cooperative Study of Retrolental Fibroplasia in 1950 looked at this relationship and found a threefold risk of ROP in infants who did not have lung disease but received prolonged oxygen supplementation.26
Other studies continued to investigate the role of oxygen in the prevention or progression of ROP.27-29 One such study, the Supplemental Therapeutic Oxygen for Prethreshold ROP (STOP-ROP) multi-center study, was designed to determine the role of oxygen in the prevention and pathogenesis of threshold ROP.26 Infants who had prethreshold ROP were randomized to conventional (89% to 94%) or supplemental (96% to 99%) oxygen for two weeks until reaching an ophthalmic endpoint, defined as development of threshold disease, full retinal vascularization, or vascularization into zone III on two sequential exams.
The investigators found that supplemental oxygen did not cause further progression of moderate ROP, but it did not significantly decrease the number of infants that would later require peripheral ablative treatment.
Steroids also have been investigated as having a role in ROP.30-33 Prenatal steroids administered to stop preterm labor have resulted in a reduction in the mortality and morbidity of infants through improved respiratory function. Certain groups have suggested that prenatal steroids are protective against ROP development.30,33 Other studies have shown that postnatal administration of steroids in the treatment of lung disease may have an adverse or no effect on the incidence of ROP.34
Light has also been studied as a contributory factor in the progression of ROP.35,37 Light-ROP was a study designed to determine the effect of decreasing light and ultraviolet light levels to which premature infants were exposed.35 The researchers randomized infants to one group that wore goggles and one group that did not. This study did not find any relationship between light exposure and the incidence or severity of ROP.37
While lower birth weight appears to be the most significant factor in the development of ROP, more research into these other environmental/biological factors may help clinicians better understand the progression of ROP and treatment options for the disease.
Examination and Treatment of ROP
Because ROP is asymptomatic, a dilated fundus exam of infants at risk for developing ROP is the only way to detect the disease. In most cases, ROP does not develop until the infant reaches 32 weeks postmenstrual age (PMA). PMA equals the gestational age of the infant plus the age after birth in weeks. Based on clinical research, the American Academy of Ophthalmology and the American Academy of Pediatrics issued a joint statement in February 2006 modifying the recommended screening of infants. The new recommendation is: Infants with a birth weight of less than 1,500g or gestational age of 30 weeks or less (as defined by the attending neonatologist) and selected infants with a birth weight between 1,500g and 2,000g or gestational age of more than 30 weeks with an unstable clinical course, including those requiring cardiorespiratory support and who are believed by their attending pediatrician or neonatologist to be at high risk, should have retinal screening examinations performed after pupillary dilation using binocular indirect ophthalmoscopy to detect ROP.36
Based on data from the CRYO-ROP and Light-ROP studies, examinations should begin by 31 weeks PMA or four weeks chronological age, whichever is later.14 Most ROP screening examinations are performed by pediatric ophthalmologists or retinal specialists. They determine the frequency of the screening examinations and/or treatment of the disease.
Frequency of the examinations for acute ROP depends on the stage seen during the initial examination. If no ROP is detected, examinations may be performed every two weeks. If ROP is detected, examinations may be performed weekly. If the clinician detects prethreshold ROP, examinations may be performed every few days. Because there is a short window of time for proper treatment, patients need to be seen in a timely manner.
Monitoring examinations for acute ROP end when either the decision for surgical intervention is made or when one of three criteria have been met:14
Zone III is completely vascularized, without previous zone I or II ROP.
The retina is completely vascularized.
The infant reaches a PMA of 45 weeks without signs of prethreshold or more severe ROP.
Because subjective observations are made on the extent of the disease, follow-up examinations should be scheduled when there is a question on vascularization or regression of ROP.
Most cases of ROP regress and do not require further management other than dilated examinations. However, some infants progress to a point at which surgical intervention is indicated.
Previously, treatment for ROP was recommended when the infant developed threshold ROP, in which the patient had a 50% chance of retinal detachment occurring.6 Clinically, threshold ROP is five contiguous or eight cumulative clock hours of stage 3 ROP in zone I or II with the presence of plus disease.
More recently, the recommendations have been modified due to the Early Treatment for Retinopathy of Prematurity Study (ETROP). Treatment is now recommended when one of the following types of high risk prethreshold ROP is seen:8
Zone I ROP, any stage, with plus disease.
Zone I ROP, stage 3 ROP, with or without plus disease.
Zone II, stage 2 or 3 ROP, with plus disease.
Treatment options include cryoablation or laser photocoagulation. Once treated, the infant is monitored closely for progression of ROP, which may warrant additional surgical procedures, or regression of the disease. Even with the proper treatment, ROP can continue to progress or can lead to visual complications that may affect the infant throughout life.
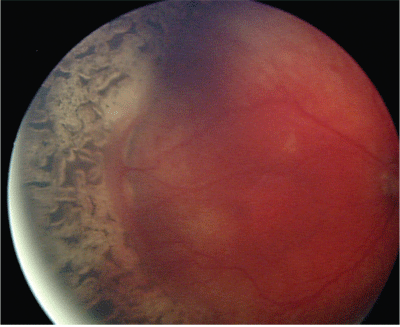 |
| Retinal appearance after laser photocoagulation for ROP. |
Ocular/Visual Complications From ROP
Surgical treatment of ROP is performed to reduce the risk of a retinal detachment in the affected eye. Even with treatment, however, there is a slight chance that a retinal detachment can develop during the patients life. Severe ROP, and in rare cases mild ROP, can be associated with other conditions, including glaucoma, cataracts, corneal opacities, microphthalmia, retinal folds, dragging of retinal vessels and macular holes.
Low birth weight, with or without ROP, can be associated with ocular complications, including myopia, strabismus and decreased contrast sensitivity. The role of myopia in low birth weight infants with and without ROP has been studied during the last few decades. Researchers have found that myopia is more prevalent in children born prematurely than in those born full term.38 The development of high myopia (>5.00D) in relation to ROP appears to be more due to changes in the anterior segment than an increase in axial length of the eye.39 Retinoscopy findings at about age 2 appear to be a good predictor for clinically significant myopia at age 10.40
Strabismus is another complication associated with premature birth. Strabismus is seen at a much higher rate in the low birth weight population with or without ROP (3% of children born full term and 20% of those born prematurely).41 More patients in this population are diagnosed with exotropia than esotropia, perhaps due to the connection between exotropia and other concurrent systemic or ocular conditions.42
A secondary complication from strabismus and possibly the refractive error is the development of amblyopia. Monitoring of suspected visual acuity differences, along with refractive error and development of strabismus, will be important to diagnose and treat amblyopia quickly.
Initially these patients are seen four to six months after they have been released from postoperative care or once the clinician feels the ROP has regressed fully. During this initial examination, it is important to get a measurement of visual acuities through age appropriate tests by using Teller cards, fixate and follow, or occlusion preference testing. The clinician will also need to evaluate binocularity through Hirschberg, Krimsky, Bruckner or simple cover test. Versions, pupils and ocular health examination including dilation are important during this examination as well. Also, it is important to get an assessment of refractive error, typically with the patient being cyclopleged. The schedule for future examinations depends on the results of this post-ROP baseline examination.
It is also important to communicate findings with the rest of the patients medical team to ensure that the patient receives the best care possible.
Retinopathy of prematurity affects thousands of children annually on a global scale. Low birth weight is the primary factor in the development of ROP; however, more research is being performed to look at the relationship other factors may have on the severity of the disease.
ROP affects the normal development of retinal vasculature and can lead to retinal detachment and blindness if left undiagnosed. However, the condition can be monitored with proper examinations and treated through surgical intervention, when indicated.
Because ROP may affect the child for the rest of his or her life, optometrists must work with other medical professionals to provide the best care for these patients. This may include performing monitoring examinations and referring patients for appropriate surgical intervention, especially in communities in which there is a need, or examining these patients, once released from follow-up, to monitor and manage ocular complications including strabismus, high refractive error and amblyopia.
Whatever role O.D.s play, we as a profession must understand ROP and the risks it poses for our patients throughout their lives. ROP may develop early in life but its impact can be felt for many decades, and we need to be ready to offer the best care we can.
Dr. Lyon is the Chief of Pediatrics and Binocular Vision Services at Indiana University School of Optometry. He previously worked in the Indiana University Pediatric Ophthalmology and Adult Strabismus clinic at Riley Childrens Hospital in Indianapolis. Dr. Warren is a former Pediatric Optometry resident at Indiana University School of Optometry. She is currently completing her Ph.D. in visual science at the school.
1. Terry TL. Extreme prematurity and fibroblastic overgrowth of persistent vascular sheath behind each crystalline lens: I, preliminary report. Am J Ophthalmol 1942;25:203-4.
2. Owens WC, Owens EU. Retrolental fibroplasia in premature infants; studies on the prophylaxis of the disease; the use of alpha tocopheryl acetate. Am J Ophthalmol 1949 Dec;32(12):1631-7.
3. Campbell K. Intensive oxygen therapy as a possible cause of retrolental fibroplasia; a clinical approach. Medical J of Australia 1951;2(2):48-50.
4. Patz A, Hoeck LE, De La Cruz E. Studies on the effect of high oxygen administration in retrolental fibroplasia: I. Nursery observations. Am J Ophthalmol 1952 Sep;35(9):1248-53.
5. National Eye Institute Web site. Retinopathy of Prematurity (ROP). Last modified Oct 2006. www.nei.nih.gov/health/rop/index.asp. (Accessed 11 October 2006.)
6. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity preliminary results. Arch Ophthalmol 1988 April; 106(4):471-9.
7. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity: ophthalmological outcomes at 10 years. Arch Ophthalmol 2001 Aug;119(8):1110-8.
8. Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol 2003 Dec; 121(12):1684-94.
9. American Academy of Ophthalmology. Ophthalmologists Warn of Shortage in Specialists Who Treat Premature Babies with Blinding Eye Condition. Press release. San Francisco, July 13, 2006. Available at www.aao.org/news/release/20060713.cfm. (Accessed 20 November 2006.)
10. Ashton N. Retinal angiogenesis in the human embryo. Br Med Bull 1970 May;26(2):103-6.
11. Roth, AM. Retinal vascular development in premature infants. Am J Ophthalmol 1977 May;84(5):636-40.
12. Hutcheson KA. Retinopathy of prematurity. Curr Opin Ophthalmol 2003 May;14(5):286-90.
13. Palmer EA, Flynn JT, Hardy RJ, et al. The incidence and early course of retinopathy of prematurity. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology 1991 Nov;98(11):1628-40.
14. Reynolds JD, Dobson V, Quinn GE, et al. Evidence-based screening criteria for retinopathy of prematurity: natural history data from the CRYO-ROP and LIGHT-ROP studies. Arch Ophthalmol 2002 Nov;120(11):1470-6.
15. Kretzer FL, Hittner HM. Retinopathy of prematurity: clinical implications of retinal development. Arch Dis Child 1988 Oct;63(10 Spec No):1151-67.
16. Stone J, Chan-Ling T, Peer J, et al. Roles of vascular endothelial growth factor and astrocyte degeneration in the genesis of retinopathy of prematurity. Invest Ophthalmol Vis Sci 1996 Feb;37(2):290-9.
17. Zhang Y, Stone J. Role of astrocytes in the control of developing retinal vessels. Invest Ophthalmol Vis Sci 1997 Sep;38(9):1653-66.
18. International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol 2005 Jul;123(7):991-9.
19. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Prognostic factors in the natural course of retinopathy of prematurity. Ophthalmology 1993 Feb;100(2): 230-7.
20. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth-factor in ocular fluid of patients with diabetic-retinopathy and other retinal disorders. N Engl J Med 1994 Dec 1;331(22):1480-7.
21. Flynn JT, Bancalari E, Snyder ES, et al. A cohort study of transcutaneous oxygen tension and the incidence and severity of retinopathy of prematurity. N Engl J Med 1992 Apr 16;326(16):1050-4.
22. Chowers I, Banin E, Hemo Y, et al. Gene transfer by viral vectors into blood vessels in a rat model of retinopathy of prematurity. Br J Ophthalmol 2001 Aug;85(8):991-5.
23. Eyetech Study Group. Anti-vascular endothelial growth factor therapy for subfoveal choroidal neovascularization secondary to age-related macular degeneration: phase II study results. Ophthalmology 2003 May;110(5):979-86.
24. McLeod DS, Taomoto M, Cao J, et al. Localization of VEGF receptor-2 (KDR/Flk-1) and effects of blocking it in oxygen-induced retinopathy. Invest Ophthalmol Vis Sci 2002 Feb;43(2):474-82.
25. Michaelson IC. The mode of development of the vascular system of the retina: with some observation on in its significance for certain retinal diseases. Trans Ophthal Soc of the UK 1948;68:137-180.
26. Kinsey VE, Jacobus JT, Hemphill F. Retrolental fibroplasias: cooperative study of retrolental fibroplasia and the use of oxygen. Arch Ophthalmol 1956;56:481-547.
27. Supplemental therapeutic oxygen for prethreshold retinopathy of prematurity (STOP-ROP), a randomized, controlled trial. I: primary outcomes. Pediatrics 2000 Feb;105(2): 295-310.
28. Katzman G, Satish M, Krishnan V. Hypoxemia and retinopathy of prematurity. Pediatrics 1987 Jun;80(6):972.
29. Kinsey VE, Arnold HJ, Kalina RE, et al. PaO2 levels and retrolental fibroplasia: a report of the cooperative study. Pediatrics 1977 Nov;60(5):655-68.
30. Higgins RD, Mendelsohn AL, DeFeo MJ, et al. Antenatal dexamethasone and decreased severity of retinopathy of prematurity. Arch Ophthalmol 1998 May;116(5):601-5.
31. Lawas-Alejo PA, Slivka S, Hernandez H, et al. Hyperoxia and glucocorticoid modify retinal vessel growth and interleukin-1 receptor antagonist in newborn rabbits. Pediatr Res 1999 Mar;45(3):313-7.
32. Penn JS, Rajaratnam VS, Collier RJ, Clark AF. The effect of an angiostatic steroid on neovascularization in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci 2001 Jan;42(1):283-90.
33. Rowlands E, Ionides AC, Chinn S, et al. Reduced incidence of retinopathy of prematurity. Br J Ophthalmol 2001 Aug;85(8):933-5.
34. Cuculich PS, DeLozier KA, Mellen BG, Shenai JP. Postnatal dexamethasone treatment and retinopathy of prematurity in very-low-birth-weight neonates. Biol Neonate 2001 Jan;79(1):9-14.
35. The design of the multicenter study of light reduction in retinopathy of prematurity (LIGHT-ROP). J Pediatr Ophthalmol Strabismus 1999 Sep-Oct;36(5):257-63.
36. Section on Ophthalmology American Academy of Pediatrics; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus. Screening examination of premature infants for retinopathy of prematurity. Pediatrics 2006 Feb;117(2):572-6. Erratum in: Pediatrics 2006 Sep;118(3):1324.
37. Reynolds JD, Hardy RJ, Kennedy KA, et al. Lack of efficacy of light reduction in preventing retinopathy of prematurity. Light Reduction in Retinopathy of Prematurity (LIGHT- ROP) Cooperative Group. N Engl J Med 1998 May 28;338 (22):1572-6.
38. Quinn GE, Dobson V, Kivlin J, et al. Prevalence of myopia between 3 months and 5 1/2 years in preterm infants with and without retinopathy of prematurity. Ophthalmology 1998 Jul;105(7):1292-300.
39. Garcia-Valenzuela E, Kaufman LM. High myopia associated with retinopathy of prematurity is primarily lenticular. J AAPOS 2005 Apr;9(2):121-8.
40. Holmstrom GE, Larsson EK. Development of spherical equivalent refraction in prematurely born children during the first 10 years of life: a population-based study. Arch Ophthalmol 2005 Oct;123(10):1404-11.
41. OConnor AR, Stephenson TJ, Johnson A, et al. Strabismus in children of birth weight less than 1701g. Arch Ophthalmol 2002 Jun;120(6):767-73.
42. Hunter DG, Ellis FJ. Prevalence of systemic and ocular disease in infantile exotropia: comparison with infantile esotropia. Ophthalmology 1999 Oct;106(10):1951-56.

