 |
Q: I have a 28-year-old graft patient following keratoconus lens intolerance who is now pregnant. She’s been on topical steroids for the past 12 months. I generally leave these patients on low-dose steroids indefinitely; however, in the case of her pregnancy, should I stop her steroids?
A: “If a patient is on topical corticosteroids once a day or every other day, then the amount of absorption of corticosteroid is so low to non-detectable that it is not of much concern,” says Kathy Tran, OD, chief of the Cornea, Contact Lenses and Refractive Surgery department at the UC Berkeley School of Optometry. “However, I still advise a patient to pinch the corner of their eyes for two minutes after administration of the medication during the first trimester,” to occlude the puncta. The concern, she says, is steroid exposure during the first six to 12 weeks of embryogenesis. “Many medications have the most serious effects on fetal development during the first trimester of pregnancy.”
There are approximately 6.5 million pregnancies annually in the United States, and approximately 64% of women are prescribed a drug during pregnancy.1,2 The FDA categorizes medications, including eye drops, according to the level of potential risk to a fetus if taken during pregnancy. Topical corticosteroids fall within Category C, Dr. Tran says, meaning studies have demonstrated an adverse effect on the fetus in animal models; data on humans is still inconclusive.
Though the original labeling system has been in place since 1979, recent changes effective June 30, 2015 now address previously missed criteria.
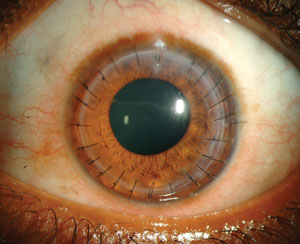 | |
| Corneal transplantation requires long-term topical steroid use to prevent rejection. But what about in patients who become pregnant? Photo: Christine W. Sindt, OD. |
James Aquavella, MD, of the University of Rochester Medical Center, notes that researchers have shown relatively frequent applications of dermatological steroid preparations in pregnant mice were followed by cleft palate changes; however, he adds that he typically prescribes one daily drop of topical steroid followed by punctal compression and has not seen any adverse effects during pregnancy.3 “My advice is to inform the patient of the risks (unknown) and benefits (i.e., reducing the incidence of graft rejection). If she agrees, continue the low-dose application,” he says. “Since most practitioners agree the risk is greater during the first trimester, an alternative would be to slowly taper and stop while observing frequently during this period.”
If the patient must use the medication three to four times per day (e.g., early in the post-op course or if the risk of graft rejection is elevated), use greater caution. Dr. Tran recommends nasolacrimal occlusion after each drop to minimize systemic absorption. “I also encourage them to drink a glass of water after each drop is administered,” she says. Some clinicians might be more comfortable inserting punctal plugs during the pregnancy to minimize absorption. Regardless of the method you choose, conduct and document a discussion with the patient’s obstetrician.
“Prescribing during pregnancy is always a risk-benefit consideration,” Dr. Tran says, who advises optometrists to “carefully weigh the benefit of treatment for any condition in a pregnant patient against the possible risks,” however remote, to the developing fetus. Discuss your concerns and offer your best clinical recommendation. If she agrees to use the medication, “document the discussion in the chart and that the benefit of the medication outweighs the risk of teratogenicity.” n
1. Ramoz LL, Patel-Shori NM. Recent changes in pregnancy and lactation labeling: retirement of risk categories. Pharmacotherapy. 2014;34(4):389-95.2. Ventura SJ, Curtin SC, Abma, JC, Henshaw SK. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990-2008. Natl Vital Stat Rep 2012;60:1-12.
3. Hemm RD, Arslanoglou L, Pollock JJ. Cleft palate following prenatal food restriction in mice: association with elevated material corticosteroids. Teratology. 1977 Jun;15(3):243-8.

