Optometric SurgeryThe scope of practice has expanded for optometrists in several states over the last few years to include minor ocular surgery and use of lasers. Today, 10 states allow ODs to perform these advanced procedures, which are on their way to becoming ingrained into the profession's DNA. Whether your state already has or has yet to expand its optometric practice scope, this month's issue of Review of Optometry intends to prepare ODs across the country to tackle these new opportunities when their time comes. Check out the other articles featured in the December issue:
|
An estimated 80 million people worldwide have glaucoma—with 95% of cases being open-angle glaucoma (OAG)—including more than three million Americans currently living with the disease. The worldwide figure is forecast to increase to over 111 million people by 2040.1 The prevalence of glaucoma increases with age in all patient populations, but African Americans and Hispanics are at higher risk overall. Its impact on patients’ quality of life, along with the associated risk of visual field loss and blindness is well-documented and can be devastating, resulting in significant costs to the healthcare system and communities at-large. The direct cost and productivity losses to the United States economy from glaucoma are estimated at a staggering $2.86 billion annually.1
On the other hand, ocular hypertension exists when a patient’s intraocular pressure (IOP) is elevated above normal (i.e. >21mm Hg), but there are no additional signs of glaucomatous damage such as progressive optic nerve cupping, retinal nerve fiber layer or ganglion cell complex thinning on optical coherence tomography (OCT) or visual field defects on automated perimetry. Ocular hypertension increases a patient’s chances of developing glaucoma; as evidenced by the Ocular Hypertension Treatment Study, which found a 49.3% 20-year cumulative incidence of OAG in one or both eyes of patients in the observation group after adjusting for exposure time.2 As a result of this risk, prophylactic treatment to lower a patient’s IOP and potentially reduce the risk for glaucoma is sometimes initiated, depending on an individual’s risk factors.
Generally speaking, in both OAG and ocular hypertension, the aqueous humor has adequate access to the angle of the eye where the trabecular meshwork (TM) is anatomically situated. In narrow-angle glaucoma (NAG), angle-closure glaucoma (ACG) and anatomically narrow angles (ANA), aqueous access to the anatomical angle of the eye is obstructed to some degree by the iris, leading to IOP increases that can be acute, chronically progressive or even cyclical in nature.
 |
|
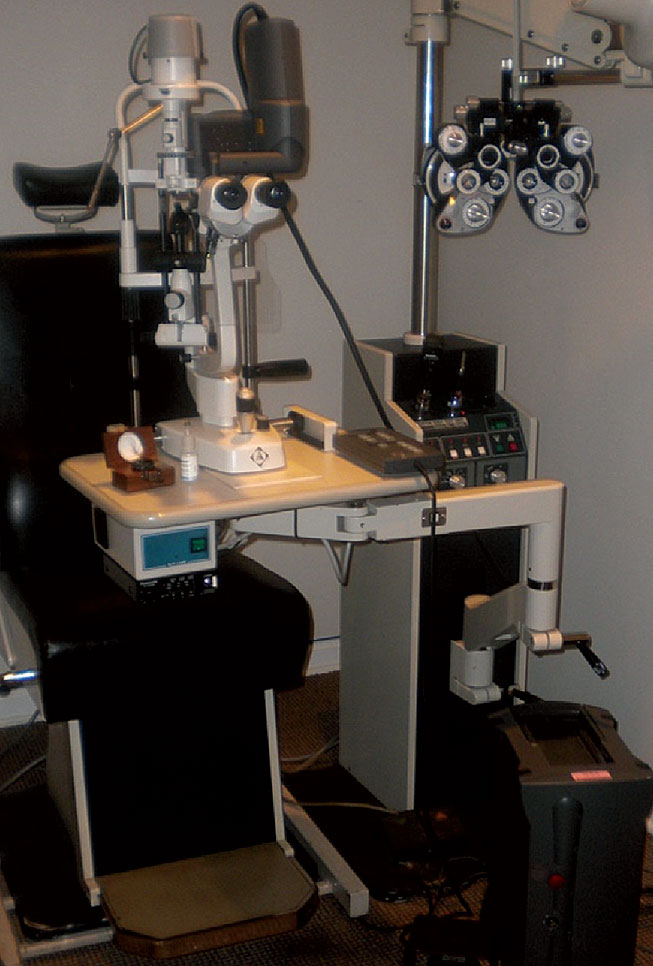
Fig. 1. An early Lumenis slit lamp-mounted SLT laser with foot pedal activation. Click image to enlarge. |
While IOP in glaucoma may be elevated or within normal ranges at baseline, the mainstay of treatment remains the reduction of IOP, which can be achieved through medical, laser and/or conventional surgical interventions. Many patients endure significant financial burdens from the cost of medications, as well as experience unwanted side effects and/or struggle with adherence to dosing regimens. This results in poorly controlled disease and creates a need for alternative therapies.
Surgical procedures for glaucoma can offer as adjunctive therapy for medically-treated glaucoma or be an alternative therapy to potentially remove those burdens. These procedures range from in-office laser procedures to operating room-based surgeries such as minimally-invasive glaucoma surgery, conventional filtering procedures and more invasive tube-shunts.
Since their inception several decades ago, laser surgeries have gained widespread acceptance, proven to be safe and efficient and have become commonplace, given they are outpatient, non-incisional and relatively quick to perform. Currently, the most commonly performed laser surgery for OAG and ocular hypertension is selective laser trabeculoplasty (SLT), while the most common laser surgery for NAG, ACG and ANA is laser peripheral iridotomy (LPI).3
 |
|
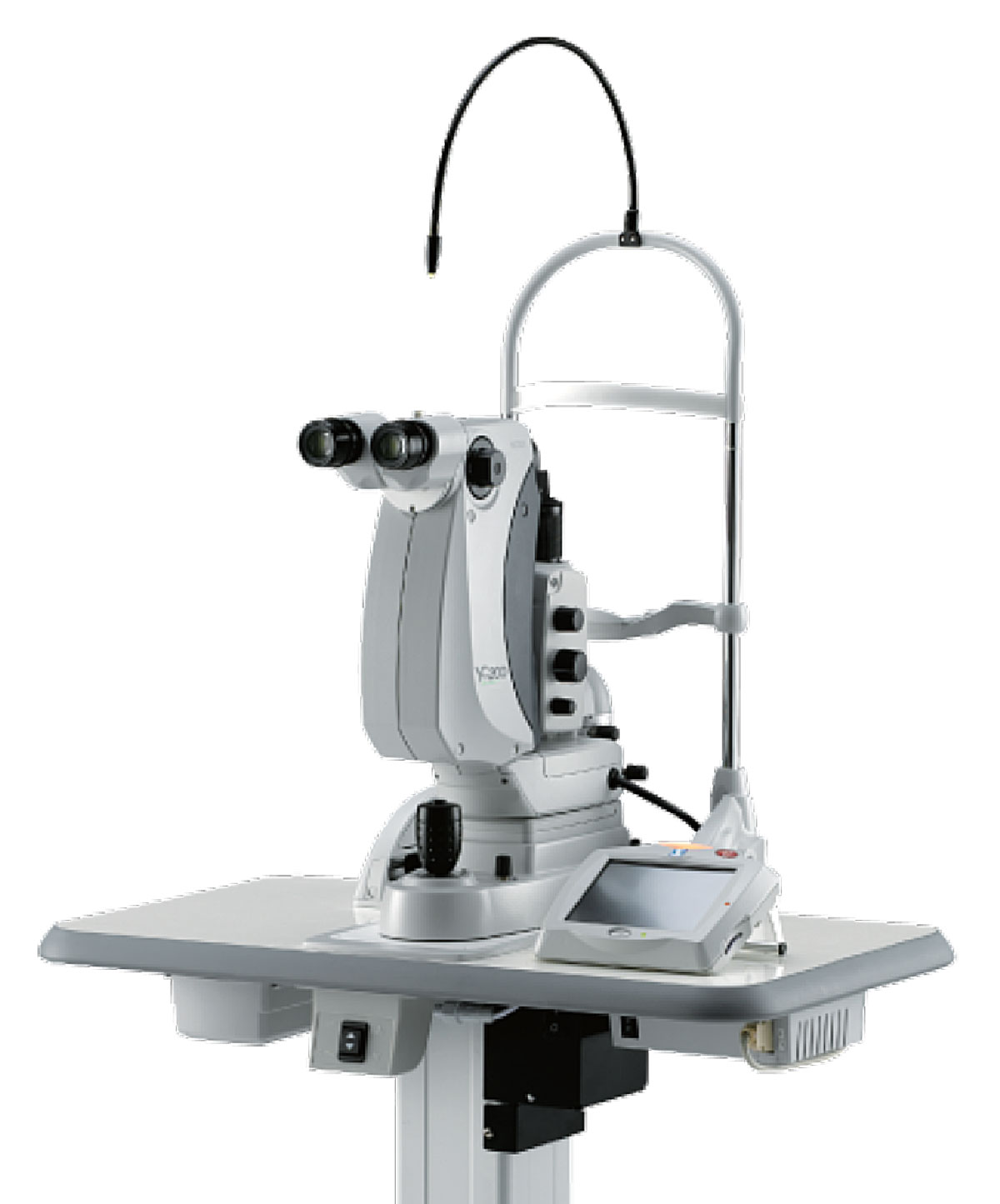
Fig. 2. A joystick-operated YAG/SLT combination laser. Photo: Nidek Ltd. Click image to enlarge. |
With numerous states in the US acknowledging the ability and skill of ODs to safely and effectively perform office-based laser surgeries—and that number is continuing to grow—thousands of patients have experienced the benefits of these procedures, with many more to come. To help get you prepared to perform these surgeries, we will give a general overview of SLT and LPI.
SLT
This procedure received approval from the Food and Drug Administration in 2001, several years after its original introduction. It quickly became the glaucoma laser surgery of choice over its predecessor, argon laser trabeculoplasty (ALT).
SLT is performed with a q-switched, frequency-doubled variation of a neodymium:yttrium-aluminum-garnet (YAG) laser, operating with a fixed spot size of 400µm, a three nanosecond pulse and a wavelength of 532nm (Figure 1).4 It is a pigment-dependent laser, meaning the light energy transmitted is preferentially absorbed by melanin pigment in the TM when employed in the angle of the eye. While it is technically a variant of a YAG laser as noted above, the SLT laser is unique and is significantly different from the traditional YAG laser, which operates as a photodisruptive laser at 1064nm with a four nanosecond pulse. Thus, a traditional YAG laser cannot be used to perform SLT, and the SLT laser cannot be used to perform YAG laser procedures (e.g., posterior capsulotomy, peripheral iridotomy). However, several laser manufacturers now offer combination YAG/SLT lasers that allow a single instrument to be switched from one laser mode to the other, in order to perform all the aforementioned surgeries (Figure 2).
 |
| Click image to enlarge. |
While several studies have found that aqueous outflow is increased by SLT, the exact mechanism of action is still not fully understood, with the first of two main theories being mechanical stretching of TM tissue resulting in an opening of outflow channels to enhance aqueous drainage, thus lowering IOP.5,6
The second theorized mechanism of action for SLT is a biologic effect that ultimately enhances aqueous outflow to reduce IOP, with clinical studies having shown SLT produces the following changes in the eye: modulating gene expression (specifically regarding cell motility, extracellular matrix production, membrane repair, and reactive oxygen species production); secreting cytokines; inducing matrix metalloproteinases; and ultimately remodeling TM.4,7,8 It is also postulated by some that the IOP-lowering effect produced by SLT may be a combination of both of these theories. Given the minimal structural damage to the TM induced by SLT, the biological theory has gained more traction and would anecdotally explain the phenomenon of a small reduction in IOP in the fellow eye often seen after the first eye undergoes SLT.
 |
|
Fig. 3. A single mirror, Latina SLT laser gonio lens with indexing bar. Click image to enlarge. |
Numerous clinical trials have proven SLT to be as effective as topical medications, resulting in overall savings to patients and to the healthcare system, as well as enhancing patient outcomes by reducing or eliminating compliance challenges encountered with daily medication dosing.4,9,10 Furthermore, SLT is accepted as a primary therapy for OAG, with studies showing an enhanced effect in treatment naïve patients, and it is becoming the initial treatment of choice for glaucoma in some countries.9,10
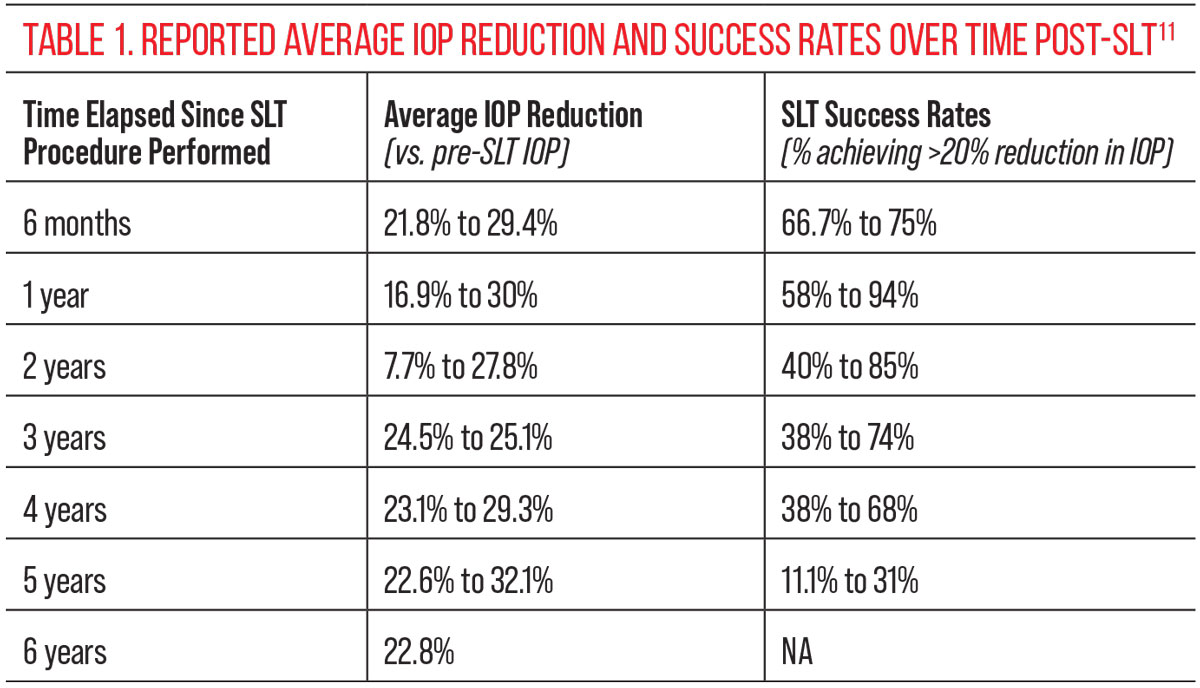
Clinical studies report various average IOP lowering effects from SLT over time, with a large range of success rates (defined as the percentage of patients achieving >20% reduction in IOP versus baseline) that all eventually wane (Table 1).11
Because the pulse duration for SLT is much shorter than ALT (three nanoseconds vs. 0.1 seconds, respectively) and the SLT laser is fundamentally different than the ALT laser, there is minimal scarring/damage to the TM. As a result, SLT is repeatable when the IOP lowering effect wears off (unlike with ALT), and it appears repeat SLT is comparable to initial SLT.4
Another potential variable in the efficacy of SLT is how much of the angle is treated. Several studies have compared 180o and 360º SLT treatment, with 360º treatment showing a slightly higher IOP reduction vs. 180º at 12 months in one case, although there was not a statistically significant difference in either study.6,12 The most commonly reported predictor of SLT success is higher baseline IOP, with some isolated studies suggesting that certain corneal biomechanical factors, as well as a pre-SLT reduction in IOP of at least 15% with topical rho-kinase (ROCK) inhibitors, may also forecast success.4,13,14
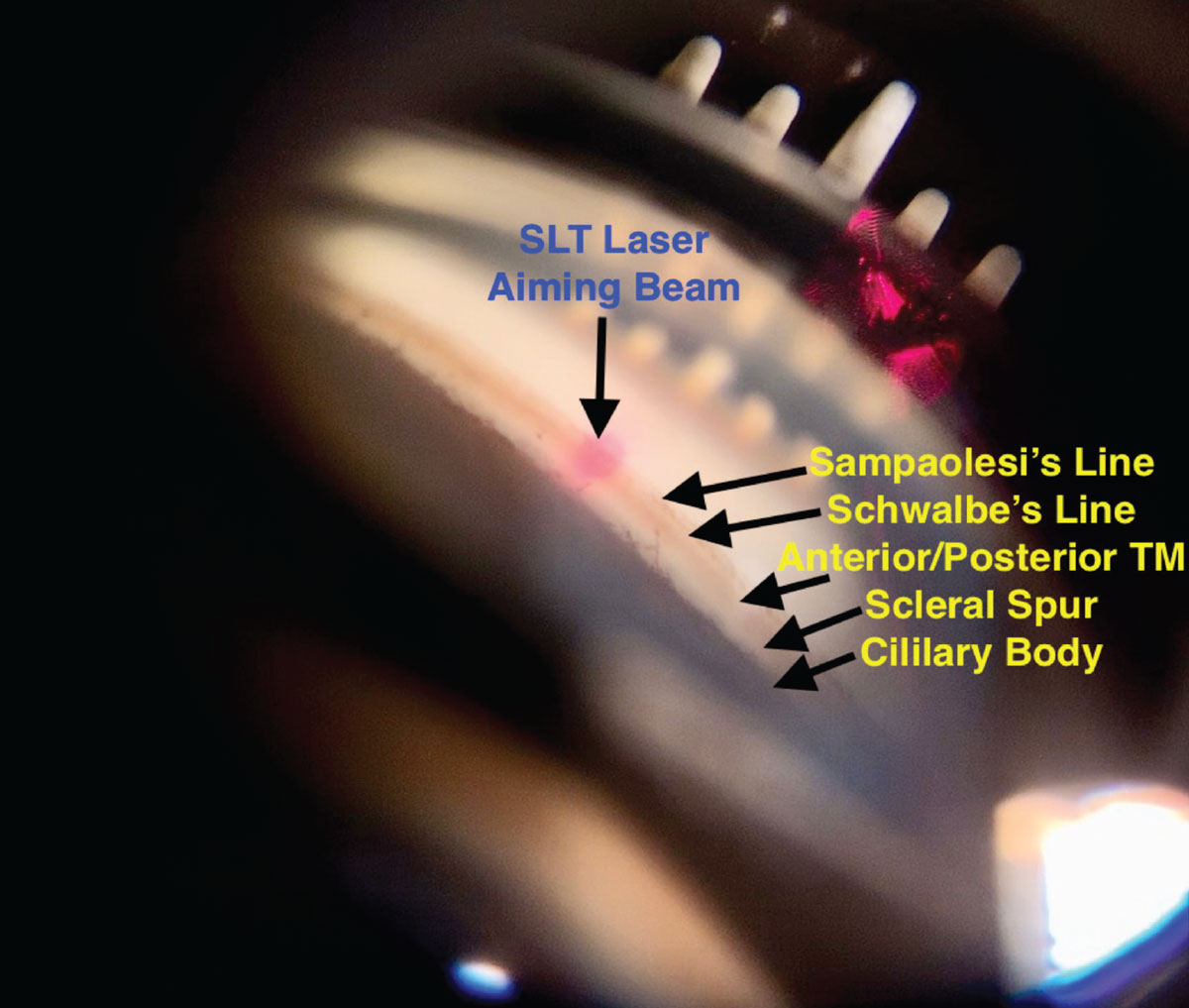 |
|
Fig. 4. A view of the angle of an eye through an SLT gonio lens, with the aiming beam focused on the trabecular meshwork. Click image to enlarge. |
Contraindications and Complications
Contraindications to SLT procedures include neovascular glaucoma, uveitic glaucoma, active intraocular inflammation, media opacities and/or narrow angles that prevent adequate visualization of the aiming beam, and/or the angle of the eye and/or poor patient cooperation. Caution should also be exercised in the presence of retinal conditions such as an epiretinal membrane, cystoid macular edema and vitreomacular traction.
Nevertheless, SLT is generally recognized as very safe, with most complications being mild and fleeting. A summary review of published SLT complications reported transient inflammation/iritis in up to 83% of patients at two to three days post-procedure; transient IOP spike of 6mm Hg at one hour postoperatively in 4.5%; corneal haze in 0.8%; only two cases of hyphema in the literature; one case of choroidal effusion; and one case of foveal burn (due to surgeon error).15
Settings and Techniques
The SLT laser uses an aiming beam to indicate where treatment is being applied, with the laser power typically initially set between 0.6mJ to 1.2mJ per shot. After informed consent is obtained and topical anesthesia is applied, topical brimonidine or equivalent is instilled to prophylactically blunt any potential IOP spike.
An SLT laser gonio lens (Figure 3) is applied to the eye using a coupling solution such as goniosol, Refresh Celluvisc Lubricant Eye Gel (Allergan) or GenTeal Lubricant Gel (Alcon). Due to mild toxicity and discomfort with goniosol, our offices have made the switch to these other coupling solutions with good results.
The aiming beams are then focused on the TM using the mirror in the gonio lens, and the procedure is commenced, applying non-overlapping laser treatment spots side-by-side for either 180º (approximately 50 shots total) or 360º (approximately 100 shots total) (Figure 4). Adequate laser-tissue interaction occurs when minimal tissue reaction is noted or very small “champagne bubbles” appear in the aqueous as the laser is fired.
Special attention should be given to the amount of pigmentation present in the TM prior to surgery. The greater the pigment level, the lower the amount of energy needed to produce the same effect, given the pigment dependent nature of the SLT laser. In some patients with heavily pigmented TM, it may be prudent to perform SLT in one quadrant (i.e., 90º) at a time to keep the risk of trabeculitis/IOP spike to a minimum. Once the procedure is completed, the lens is removed, the eye is rinsed, a second drop of brimonidine is applied and IOP should be assessed in one hour, with any spike treated appropriately.
A non-steroidal drop can be prescribed for the immediate post-op period if needed. Follow-up to assess IOP is usually scheduled within several weeks, keeping in mind there is generally a 10-day global period for SLT with many insurance carriers.
LPI
This surgery is employed urgently to emergently when a patient experiences acute angle closure (i.e., the iris is positioned anteriorly such that aqueous access to the TM is completely obstructed) or when a patient with NAG or ANA—including plateau iris syndrome—is deemed to be at high-risk for angle closure.
For ACG and AAC, LPI generally should not be performed until IOP and any associated inflammation are first controlled.16 Keep in mind that hyperopes tend to have shallower anterior chamber angles, and gonioscopy should be performed on all patients prior to LPI. Further, studies indicate a 50% risk of developing AAC in the fellow eye for patients experiencing monocular AAC.17
LPI creates an opening in the peripheral iris, relieving pupillary block and allowing aqueous to bypass the traditional transpupillary drainage route and directly enter the angle of the eye. An argon or photocoagulative laser may be used by itself to perform LPI, offering a beneficial hemostatic effect (making it less likely to cause hyphema), but it takes longer to perform the procedure and this laser is less commonly found in eye care office settings. Combination LPI procedures can be performed in which an argon laser is used to ablate most of the iris, with a YAG laser then employed to finish the iridotomy; however, this requires the purchase of two separate lasers. Thus, the YAG laser, (a pigment independent, photodisruptive laser without hemostatic effect), is most commonly used by itself to perform LPI (Figure 5).
 |
Fig. 5. A YAG laser is most commonly used to perform LPI. Pictured here is a Nidek standalone laser. Click image to enlarge. |
A YAG laser operates at a wavelength of 1064nm and causes a focal, 27,000° F increase in temperature within its four nanosecond energy pulse. Since its wavelength is beyond the visible light spectrum of 300nnm to 700nm, a focusing beam (commonly comprised of helium and neon with a wavelength of 633nm) is used to visualize the focal plane of the laser. The focusing beam consists of two separate circles that, when brought together, indicate the location of the laser’s focal plane.
Contraindications and Complications
Contraindications include active intraocular inflammation, media opacities preventing adequate visualization of the focusing beam and/or the iris, poor patient cooperation and synechial angle closure from neovascular glaucoma or iridocorneal endothelial syndrome.16 Caution should also be exercised in the presence of retinal conditions such as epiretinal membrane, cystoid macular edema and vitreomacular traction.
 |
|
Fig. 6. An Abraham iridotomy YAG laser lens. Note the magnifying “button” that is offset in the lens. Click image to enlarge. |
Possible complications include transient IOP spike (in up to 70% of patients), uveitis, iris bleeding/hyphema (in up to 50% of patients), corectopia, focal cataract, corneal burns, posterior synechiae and subjective visual dysphotopsias.18-26 Studies have hypothesized that these dysphtopsias may be the result of iridotomy position in relation to the lid and/or from the tear prism at the lid margin refracting light through the iridotomy. Clinical studies are contradictory as to the best location for LPI to minimize dysphotopsia risk, with some advocating for 3 o’clock or 9 o’clock, and others for 11 o’clock or 1 o’clock, but all agree that the vast majority of LPI patients will not experience dysphotopsia.27-29 In patients with uveitis who require LPI, there is also an increased risk of iridotomy closure.21
Settings and Techniques
For LPI, the YAG power is typically set between 3.0mJ to 4.0mJ per pulse to start, with a single, double or triple-burst setting, depending on surgeon preference. Most YAG lasers have variable anterior, posterior or zero-offset settings to account for the shockwave of energy that travels back toward the surgeon when the laser is fired. The laser offset is most commonly set to zero for LPI (or at a small posterior offset). A laser lens designed for LPI that has a magnifying “button” to concentrate laser energy and magnify the iris (Figure 6) is applied to the eye using a coupling solution after topical anesthesia and is applied and once informated consent obtained.
While a laser lens is optional when performing YAG capsulotomy, it is used for LPI because of the aforementioned benefits, as well as offering the ability to use the lens to apply gentle pressure to the eye for 10 to 20 seconds to staunch the bleeding should it occur. Any resulting hyphema is treated accordingly.
Topical pilocarpine 1% may be instilled pre-LPI to induce miosis, thereby thinning the iris as it stretches in hopes of reducing the number of laser shots and total amount of laser energy needed to accomplish the iridotomy. When using pilocarpine preoperatively, it is helpful to advise the patient of the potential for brow ache after administration. Topical brimonidine (or a similar non-inflammatory ocular hypotensive) is also instilled pre- and immediately postoperatively to blunt potential IOP spikes.
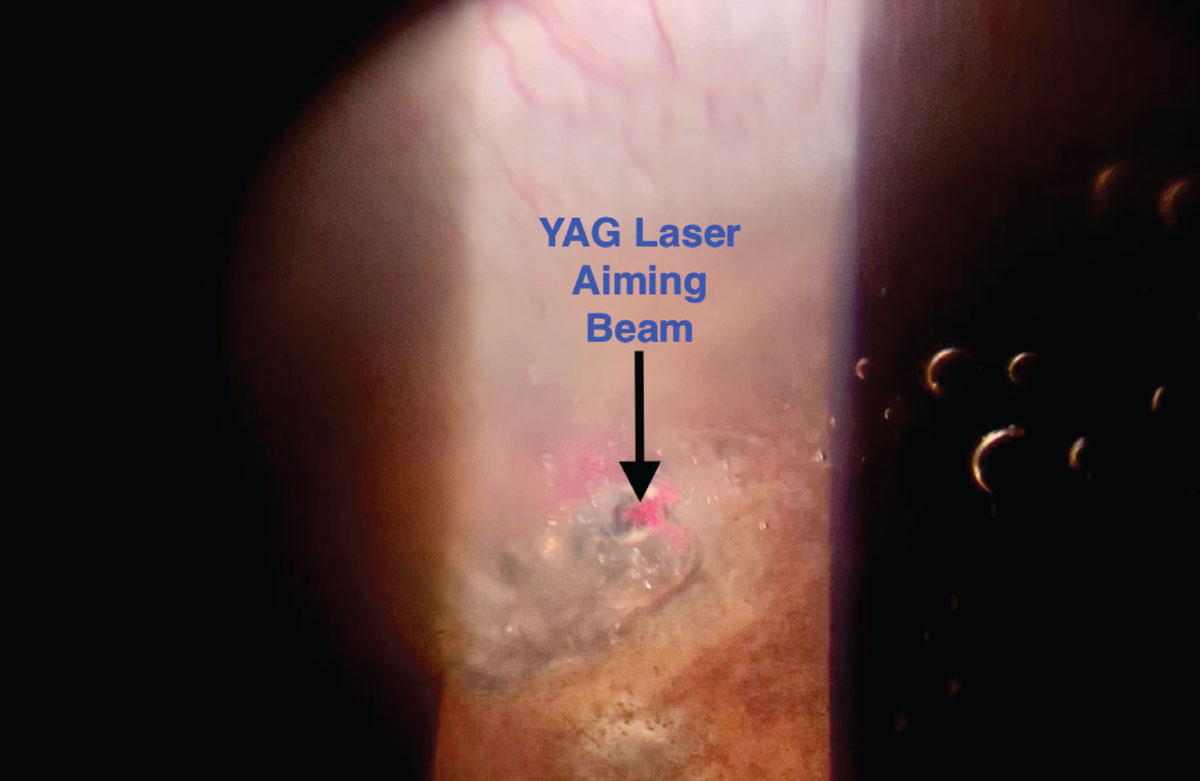 |
|
Fig. 7. This is a view of a YAG laser aiming beam focused on an iris crypt with early plume of aqueous into the anterior chamber during LPI. Also note the bubbles and debris beginning to cloud the view. Click image to enlarge. |
An iris “crypt” (i.e., an area of thinner iris) located approximately one-third of the distance from the angle of the eye to the pupillary margin is an ideal site for an iridotomy. LPI has traditionally avoided the 12 o’clock position in the eye because the bubbles and debris that are liberated from the iris during LPI are lighter than aqueous, causing them to float to the 12:00 position and potentially obscure the view for subsequent laser shots. Regardless of the location at which LPI is performed, it can create a cloudy or hazy view during the procedure.
The surgery is continued until a characteristic “plume” of aqueous gushes into the anterior chamber, at which time a few more laser shots are applied to enlarge the LPI to about 0.5mm to 1mm in diameter (Figure 7). After post-op brimonidine is instilled, IOP is re-checked about an hour later. IOP spikes are treated accordingly and patients are typically discharged with a topical steroid dosed QID to prevent iridotomy closure and synechia.
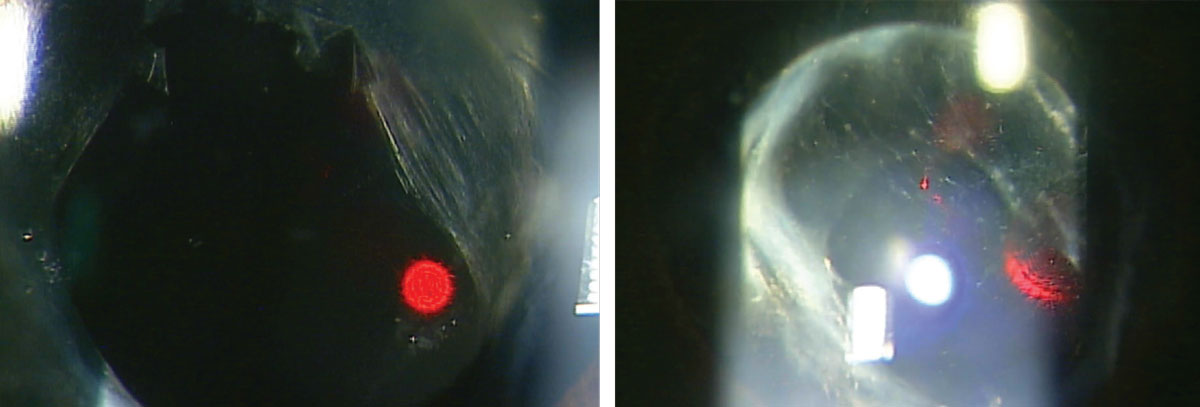 |
A patient before (left) and after (right) undergoing a YAG posterior capsulotomy. Photos: Nate Lighthizer, OD. Click image to enlarge. |
Postoperative follow-up is generally one week (to assess IOP, irdotomy patency and gonioscopy for synechia formation, while hopefully confirming a widening of the anterior chamber angle) and at one month (to include dilated fundus exam).
Takeaways
Our understanding of glaucoma, the ability to diagnose it earlier and our options for effective treatment have never been greater than they are today. From new and emerging pharmaceuticals to alternative drug delivery technologies, evolving non-traditional therapies and cell and gene therapy research to current and still developing surgical advances including lasers and MIGS procedures, our opportunities to prevent vison loss for patients with glaucoma continues to exponentially expand.
Perhaps one day in the not-too-distant future we will even be able to restore vision that has been lost to glaucoma. In the meantime, SLT and LPI remain effective therapies for various glaucomatous conditions, offering numerous clinical benefits with favorable safety profiles, all while being performed in outpatient or office-based settings.
As optometric scope of practice continues to expand nationally and optometric education continues to teach, train and prepare current and future doctors of optometry, even greater access to these laser procedures will be afforded to patients. The future of effective glaucoma management is indeed bright for patients and doctors alike.
Dr. Anatasio practices full scope optometry at his practice, Louisiana Family Eyecare. He also lectures and has published articles and posters on optometric surgery. Dr. Wroten is chief operating officer at the Bond-Wroten Eye Clinics in southeast Louisiana, where he supervises its residency in primary care and ocular disease. He has served in a number of different capacities within the profession, lectured internationally and published numerous articles on a variety of topics in eye care. They have no financial disclosures.
1. Glaucoma: Facts and Figures. National Glaucoma Research program of the BrightFocus Foundation. https://www.brightfocus.org/glaucoma/article/glaucoma-facts-figures. July 14, 2021. Accessed October 1, 2022. 2. Kass MA, Heuer DK, Higginbotham EJ, et al. Assessment of cumulative incidence and severity of primary open-angle glaucoma among participants in the ocular hypertension treatment study after 20 years of follow-up. JAMA Ophthalmol. 2021;139(5):1-9. 3. Arora KS, Robin AL, Corcoran KJ, et al. Use of various glaucoma surgeries and procedures in medicare beneficiaries from 1994 to 2012. Ophthalmology. 2015;122(8):1615-24. 4. Garg A, Gazzard G. Selective laser trabeculoplasty: past, present, and future. Eye (Lond). 2018;32(5):863-76. 5. Gulati V, Fan S, Gardner BJ, et al. Mechanism of action of selective laser trabeculoplasty and predictors of response. Invest Ophthalmol Vis Sci. 2017;58:1462-8. 6. Goyal S, Beltran-Agullo L, Rashid S, et al. Effect of primary selective laser trabeculoplasty on tonographic outflow facility: a randomised clinical trial. Br J Ophthalmol. 2010;94(11):1443-7. 7. Kagan DB, Gorfinkel NS, Hutnik CM. Mechanisms of selective laser trabeculoplasty: a review. Clin Exp Ophthalmol. 2014;42(7):675-81. 8. Izzotti A, Longobardi M, Cartiglia C, et al. Trabecular meshwork gene expression after selective laser trabeculoplasty. PLoS One. 2011;6(7):e20110. 9. DeKeyser M, DeBelder M, Debelder S, DeGroot V. Where does selective laser trabeculoplasty stand now? A review. Eye Vis (Lond). 2016;3:10. 10. Konstantakopoulou E, Gazzard G. Is selective laser trabeculoplasty shifting the glaucoma treatment paradigm in developing countries? Brit Jour Ophthalmol. 2022;106:1185-6. 11. Leahy KE, White AJ. Selective laser trabeculoplasty: current perspectives. Clin Ophthalmol. 2015;9:833-41. 12. Nagar M, Ogunyomade A, O'Brart DP, et al. A randomised, prospective study comparing selective laser trabeculoplasty with latanoprost for the control of intraocular pressure in ocular hypertension and open angle glaucoma. Br J Ophthalmol. 2005;89(11):1413-7. 13. Hirneib C, Sekura K, Brandlhuber U, Kampik A, Kernt M. Corneal biomechanics predict the outcome of selective laser trabeculoplasty in medically uncontrolled glaucoma. Graefes Arch Clin Exp Ophthalmol. 2013;251(10):2382-8. 14. Baba T, Hirooka K, Nii H, Kiuchi Y. Responsiveness to ripasudil may be a potential outcome marker for selective laser trabeculoplasty in patients with primary open-angle glaucoma. Scientific Reports. 2021;11:5812. 15. Song J. Complications of selective laser trabeculoplasty: a review. Clin Ophthalmol 2016;10:137-43. 16. Cao FX. Peripheral iridotomy. Medscape. https://emedicine.medscape.com/article/1844179-overview. Updated: June 15, 2020. Accessed Oct. 1, 2022. 17. Shaarawy TM, Sherwood MB, Hitchings RA, Crowston JG. Glaucoma, 2nd ed. China: Saunders Ltd.; 2009. 18. Moster MR, Schwartz LW, Spaeth GL, et al. Laser iridectomy. A controlled study comparing argon and neodymium: YAG. Ophthalmology. 1986;93(1):20-4. 19. Pollack IP, Robin AL, Dragon DM, et al. Use of the neodymium:YAG laser to create iridotomies in monkeys and humans. Trans Am Ophthalmol Soc. 1984;82:307-28. 20. Krupin T, Stone RA, Cohen BH, et al. Acute intraocular pressure response to argon laser iridotomy. Ophthalmology. 1985;92(7):922-6. 21. Ritch R, Liebmann JM. Laser iridotomy and peripheral iridoplasty. In: Ritch R, Shields MB, Krupin T. The Glaucomas. 2nd ed. St. Louis, MO: CV Mosby; 1996. 22. McAllister JA, Schwartz LW, Moster M, Spaeth GL. Laser peripheral iridectomy comparing Q-switched neodymium YAG with argon. Trans Ophthalmol Soc U K. 1985;104(Pt 1):67-9. 23. Gilbert CM, Robin AL, Pollack IP. Iridotomy using the Q-switched neodymium (Nd):YAG laser. Ophthalmology. 1984;91(9):1123. 24. Robin AL, Eliassi-Rad B. Laser Iridotomy. Morrison JC, Pollack IP. Glaucoma: Science and Practice. New York, NY: Thieme. 2003. 25. Vijaya L, Asokan R, Panday M, George R. Is prophylactic laser peripheral iridotomy for primary angle closure suspects a risk factor for cataract progression? The Chennai Eye Disease Incidence Study. Br J Ophthalmol. 2017;101(5):665-70. 26. Lederer CM Jr, Price PK. Posterior synechiae after laser iridectomy. Ann Ophthalmol. 1989;21(2):61-4. 27. Spaeth GL, Idowu O, Seligsohn A, et al. The effects of iridotomy size and position on symptoms following laser peripheral iridotomy. J Glaucoma. 2005;14(5):364-7. 28. Vera V, Naqi A, Belovay FW, et al. Dysphotopsia after temporal versus superior laser peripheral iridotomy: a prospective randomized paired eye trial. Am J Ophthalmol. 2014;157(5):929-35. 29. Srinivasan K, Zebardast N, Krishnamurthy P, et al. Comparison of new visual disturbances after superior vs. nasal/temporal laser peripheral iridotomy. Ophthalmology. 2018;125(3):345-51. |