The management of bacterial eye infections was revolutionized by the development of topical fluoroquinolones. All fluoroquinolones have an FDA indication for the treatment of bacterial conjunctivitis. In 2004, Iquix (1.5% levofloxacin, Vistakon), a third-generation agent, received an additional FDA approval for the treatment of bacterial corneal ulcers. Since then, fourth-generation fluoroquinolones evolved with varying concentrations and dosing (see “Commonly Prescribed Topical Fluoroquinolones,” below).
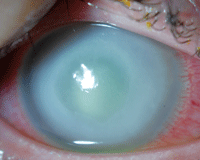
This patient presented with a pronounced Pseudomonas ulcer. What is the most effective treatment strategy?
Nonetheless, all newer generation fluoroquinolones are used off-label for numerous conditions from ulcerative keratitis to infection prevention in pre- and post-surgical care. Looking back over the last 20 years, we must ask if these drugs are living up to their promise and what will the future of ocular infection management and prevention look like? Regardless, our biggest concern going forward is the development of emerging resistance patterns to topical fluoroquinolones.
Mechanism of Action
Topical fluoroquinolones are antibiotic agents that block bacterial DNA synthesis by inhibiting the topoisomerase enzymes. Older-generation topical fluoroquinolones (ciprofloxacin, ofloxacin and levofloxacin) specifically target the DNA enzyme topoisomerase IV, which is more susceptible in gram-negative bacteria.1,2 However, the fourth-generation fluoroquinolones (moxifloxacin, gatifloxacin) contain a substitution of a methoxy group at position eight of the quinolone ring, which additionally facilitates topoisomerase II (DNA gyrase) inhibition in gram-positive organisms.
This dual mechanism of action promotes increased bactericidal activity against gram-positive organisms without sacrificing the gram-negative coverage provided by older-generation fluoroquinolones.3 Most importantly, targeting both topoisomerase II and IV likely reduces resistance because concomitant mutations in both genes are much less likely to occur than a single mutation in one gene.4,5
Drug Penetration and Tissue Concentration
For topical fluoroquinolones to be effective in the treatment of ocular infections, they must exhibit satisfactory minimum inhibitory concentration (MIC) scores, high penetration rates into ocular tissues/aqueous and broad susceptibility profiles, as well as posess the ability to thwart resistance.
The MIC is defined as the lowest possible concentration of an antibiotic that is able to prevent overnight micro-organism growth in a tissue culture. Typically, MIC90 (the MIC score required to inhibit bacterial growth in at least 90% of targeted strains) is the standard measure of bactericidal efficacy for topical anti-infective agents. Note that the lower the MIC90 value, the more potent the agent is against a given isolate.
The ocular surface poses a challenge to drug administration and pharmacokinetics. Tears act as a drug-delivery barrier, effectively diluting the agent and facilitating drainage via the nasolacrimal system upon rapid blinking following instillation.6 Therefore, MIC scores derived from in vitro laboratory testing may not accurately reflect on-eye MIC values. Delivering the drug to the target tissue, as well as remaining there long enough to be effective in vivo, is of equal importance. Compared to their systemic counterparts, topical antibiotics often must be dosed at higher concentrations to increase drug penetration and sustain effective therapeutic levels.
The fourth-generation fluoroquinolones offer the best penetration profile; in particular, moxifloxacin and 1.5% levofloxacin fared well in corneal penetration studies.7-9, 15-18
Drug Enhancements
In an effort to amplify antibiotic concentration on the ocular surface and improve penetration, many ocular pharmaceutical companies developed enhanced or reformulated versions of existing fluoroquinolones during the last eight years. For example, Vistakon released Iquix in 2004, which contained more than double the concentration of levofloxacin in Quixin.10 Also, in May 2010, Allergan brought Zymaxid to the market, which contained 0.2% more gatifloxacin than Zymar.11
Commonly Prescribed Topical Fluoroquinolones
Generic Name
Brand Name and Approval Date
Manufacturer
Classification
Indication
0.3% Ciprofloxacin
Ciloxan,
December 1990 Alcon, generic
2nd Generation
Bacterial Conjunctivitis
0.3% Ofloxacin
Ocuflox,
July 1993
Allergan, generic
2nd Generation
Bacterial Conjunctivitis
0.5% Levofloxacin
Quixin,
August 2000Vistakon
3rd Generation
Bacterial Conjunctivitis
0.3% Gatifloxacin
Zymar,
March 2003
Allergan
4th Generation
Bacterial Conjunctivitis
0.5% Moxifloxacin
Vigamox,
April 2003
Alcon
4th Generation
Bacterial Conjunctivitis
1.5% Levofloxacin
Iquix,
March 2004Vistakon
3rd Generation
Bacterial Corneal Ulcers
0.6% Besifloxacin
Besivance,
May 2009
Bausch + Lomb
Novel (no systemic equivalent)
Bacterial Conjunctivitis
0.5% Gatifloxacin
Zymaxid,
May 2010Allergan
4th Generation
Bacterial Conjunctivitis
0.5% Moxifloxacin
Moxeza, November 2010
Alcon
4th Generation
Bacterial Conjunctivitis
Then, in November 2010, Alcon released Moxeza as a successor to Vigamox. In this instance, the concentration of moxifloxacin was not increased. Instead, the company reformulated Vigamox with a new delivery vehicle, xanthan gum. In clinical trials, the xanthan gum facilitated increased corneal penetration of moxifloxacin. Most significantly, the increased drug penetration of Moxeza permitted b.i.d. dosing vs. t.i.d. dosing for Vigamox.12
Bausch + Lomb’s release of Besivance (besifloxacin) in 2009 was a significant development in ocular antibiotics. Besifloxacin was developed for the sole purpose of ophthalmic use, and has no systemic counterpart.13 This unique aspect could potentially help minimize resistance to the drug over the long term. The drug features a unique chlorine group located at position eight of the quinolone ring, as well as an aminoazepinyl component at the C-7 location, which may enhance activity against gram-positive bacteria.14
Additionally, Besivance uses the DuraSite vehicle (InSite Vision), which prolongs the drug’s contact time on the ocular surface and enhances corneal penetration. Finally, it is important to note that Besivance just received an additional FDA indication for the treatment of conjunctivitis associated with Pseudomonas aeruginosa in September 2012.
The Effect of BAK
For many years, researchers have debated the potential benefits and limitations of the preservative benzalkonium chloride (BAK) in topical antibiotics. In topical medications that are commonly used long-term, such as glaucoma agents, the presence of BAK has been shown to cause ocular surface inflammation and corneal damage.15 However, it has been suggested that, because BAK disrupts the ocular surface, it actually may enhance corneal penetration of the drug. Furthermore, BAK has been shown to increase epithelial permeability and trans-scleral drug delivery.16 Currently, Vigamox, Moxeza and Iquix are the few topical fluoroquinolones that do not contain BAK.
Interestingly, some studies indicated that BAK-preserved Zymar is more effective at targeting methicillin-resistant Staphylococcus aureus (MRSA) than BAK-free Vigamox in vitro.17 Other in vitro research suggested that, while BAK may enhance an antibiotic’s effect, it is cleared by the tears rapidly.18 More specifically, the study author noted that the concentration of BAK in the tears is undetectable five minutes after installation.18
Bactericidal Resistance
In addition to corneal penetration, pharmacodynamics (e.g., kill rates, resistance patterns and susceptibility profiles) is another crucial aspect of topical fluoroquinolones. Studies suggest that MICs for fourth-generation fluoroquinolones are lower (better) than second-generation fluoroquinolones for all gram-positive organisms.19,20 The MIC90 for ciprofloxacin was lower in gram-negative bacteria compared to the newer-generation fluoroquinolones, and was the most effective against Pseudomonas among all topical fluoroquinolones tested.19,20
Bactericidal resistance is a result of many factors, including overprescribing and negligent prescribing of antibiotics (e.g., errant use of antibiotics for a viral condition), improper dosing (including patient non-compliance), and widespread use of antibiotics in the livestock industry. Infection by drug-resistant organisms such as MRSA and methicillin-resistant Staphylococcus epidermidis (MRSE) is a growing concern in the ophthalmic community. According to the Centers for Disease Control and Prevention, 1.5% of the population (4 million Americans) are MRSA carriers.21 Patients in nursing care facilities, health care workers, and those who live in communities where skin-to-skin contact is prevalent are at higher risk for MRSA infections.21
The Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) study was designed to monitor antibiotic susceptibility trends in ocular isolates.22 The results indicated that 39% of S. aureus ocular isolates were methicillin resistant and 38% were fluoroquinolone resistant. Among the coagulase-negative staphylococci isolates sampled from ocular infections, 53% were methicillin resistant and 43% were fluoroquinolone resistant.22 This is similar to the original data collected by The Surveillance Network (TSN) from 2001 to 2006, which revealed multidrug-resistant MRSA in ocular isolates.23 Of interest, TSN researchers noted that trimethoprim was the most effective agent against MRSA.23
Currently, the Ocular Tracking Resistance in the United States Today (TRUST) program is the largest annual, longitudinal, nationwide surveillance of antibiotic resistance. Since the program’s launch in 2005, Ocular TRUST researchers have evaluated the in vitro susceptibility of Staph., Strep., and Haemophilus ocular isolates to numerous antibiotics.24 The most recent data from Ocular TRUST suggests that just 15% to 30% of MRSA isolates are susceptible to currently available topical fluoroquinolones (Besivance was not evaluated, because it was not yet FDA approved at study initiation).24
The combined results from ARMOR, TSN and Ocular TRUST show that resistance to multiple antibiotics is prevalent among ocular bacterial pathogens. Again, keep in mind that in vitro resistance does not necessarily equate to in vivo resistance. Still, eye care clinicians must consider the potential danger associated with such expanding resistance patterns.
• Don’t underdose an active infection.
Antibiotics with the lowest MIC values and the highest delivery
concentration are best suited to eradicate ocular infections. For active
infections, dose antibiotics at their recommended frequency to
eliminate the bacteria quickly. The greatest risk for the development of
antibiotic resistance comes from sustained sub-therapeutic dosing,
which doesn’t eradicate the infection but potentially affords it time to
mutate.
• Be judicious in
prophylactic use. When used for surgical prophylaxis, antibiotics can
be discontinued sooner than in cases of active infection. A recent study
found that repeated fluoroquinolone use after intravitreal injection
led to increased rates of resistance.30 So, instead of using
prophylactic antibiotics, many retinal specialists now are using
facemasks and iodine/aseptic techniques during injection to minimize the
risks of infection. For infection prevention in cataract surgery, good
wound architecture is critical. Today, with femtosecond laser-assisted
surgery, wound leaks and consequent endophthalmitis risks may be less
likely. • Avoid
overprescribing. Improper diagnosis of red eye is a specific instance
when topical antibiotics frequently are overprescribed. Too often,
pediatricians, urgent care physicians and even
optometrists/ophthalmologists prescribe antibiotics for conditions other
than bacterial conjunctivitis, when the diagnosis is not clear.
Overprescribing propagates bactericidal resistance, so exercise good
clinical judgment when using an antibiotic.
•
Choose an appropriate antibiotic. Consider reserving a “big gun”
antibiotic, such as a fourth-generation fluoroquinolone, for the most
serious infectious cases. Second-generation fluoroquinolones,
aminoglycosides or azithromycin (Azasite, Merck) are alternative
antibiotic choices for infections that are not sight threatening. Such
alternatives may be effective, and choosing them could potentially limit
fourth-generation antibiotic resistance. Also, review the FDA drug
safety classification for children and pregnant patients. For example,
Azasite may be a good choice for pregnant or nursing mothers because it
is a Category B drug. • When in
doubt, culture. Smears and cultures are recommended in cases that
involve a large infiltrate (>3mm); are chronic in nature or appear
unresponsive to broad-spectrum antibiotic therapy; or that exhibit
atypical clinical features indicative of fungal, amoebic or
Mycobacterium keratitis. The sensitivities from cultures can help guide
appropriate antibiotic selection. In
cases of suspected bacterial keratitis, fourth-generation
fluoroquinolones routinely are used off-label and are widely accepted as
first-line therapy. Depending on its location and severity, microbial
keratitis can be a sight-threatening condition. For central or severe
keratitis, a loading dose (q15m for the first hour) followed by frequent
applications (q1h around the clock) is recommended.
For
drug-resistant pathogens, such as MRSA or MRSE, fluoroquinolones may
not prove effective. If a bacterial infection is not clinically
responsive to aggressive treatment with a fluoroquinolone, suspect
MRSA/MRSE. Consider treatment with fortified vancomycin, trimethoprim or
sulfacetamide.31 In these instances, be certain to obtain cultures in
an effort to select the most effective antibiotic. Additional
therapeutic options that may be effective against MRSA/MRSE include
bacitracin ointment, cefazolin injection and oral linezolid. Concurrent
use of oral rifampin and linezolid have been shown to be more effective
in the treatment of preseptal cellulitis/cutaneous infections than
intravenous vancomycin.32
•
Be wary of Mycobacterium. Regarding refractive surgery, infection rates
fortunately are low. But, if an infection occurs, atypical
Mycobacterium infiltrates may be of greatest concern. Although rare,
Mycobacterium are found in ultrasound water baths used for instrument
processing or in surgical field moisture. In any confirmed case of
Mycobacterium chelonae infection, researchers at Johns Hopkins recommend
500mg clarithromycin b.i.d., topical tobramycin and a fourth-generation
fluoroquinolone.33
•
Consider the cost. Because of rising medication prices, patients are
sometimes forced to use generic antibiotics in lieu of branded
medications. Newer-generation fluoroquinolones have the broadest
antimicrobial spectrum. But, if patients cannot afford these, which
generic agent is best? To date, no studies offer a real solution to this
question, and practitioners are faced with the ongoing dilemma of cost
vs. efficacy.
However,
generic trimethoprim-polymyxin B should be considered when there is a
risk of MRSA. Despite being an older drug, more than 90% of MRSA
isolates were susceptible to trimethoprim in vitro.28 Also,
the second-generation fluoroquinolones are cost effective, and have a
broad spectrum of coverage. They are a good choice if a generic is
needed. Regardless, educate patients about the potential risks
associated with having to choose between the lower cost of generic
medications and the therapeutic superiority of branded drugs.
Recommendations for Fluoroquinolone Use
Use in Surgical Prophylaxis
Topical antibiotics are used routinely for infection prevention in all forms of ocular surgery. The discussion about appropriate use of prophylactic antibiotics is a hot topic among eye care professionals due to increased resistance patterns. Some studies have reported increased endophthalmitis rates since 2000 despite prophylactic antibiotic use.25
There are several possible reasons why endophthalmitis is on the rise. For example, more surgeons perform cataract surgery using sutureless, clear corneal incisions, which are more prone to leakage. Additionally, intravitreal anti-VEGF and steroidal injections are being administered more frequently for the treatment of macular degeneration and diabetic eye disease.
Most cases of postoperative endophthalmitis are due to S. epidermidis, followed by S. aureus.26 Further, epidemiologic evidence suggests that MRSA is responsible for approximately 3% of all endophthalmitis cases.26
The overall rate of endophthalmitis after uncomplicated cataract surgery in patients who received prophylactic treatment with gatifloxacin and moxifloxacin was 0.07%.26 Another study found a similar endophthalmitis rate for those who received gatifloxacin and moxifloxacin (0.056%), but a higher incidence in patients who were treated with ciprofloxacin and ofloxacin (0.197%).27
These data suggest that the fourth-generation fluoroquinolones are more effective against gram-positive organisms and may be the superior choice for surgical endophthalmitis prevention. Fortunately, the risk of post-surgical infection is extremely rare. And ultimately, the low rates of endophthalmitis also make it exceedingly difficult to compare the prophylactic efficacy of different antibiotics exhaustively.
During the last few years, intracameral antibiotics have been evaluated for use in ocular surgery. A recent study by the European Society of Cataract & Refractive Surgeons (ESCRS) showed that cefuroxime injection can reduce endophthalmitis risk in cataract surgery.28 Further, intracameral injections have the potential to help reduce postoperative patient noncompliance/drop confusion, and could be more cost effective than topical anti-infective use.
The primary barrier to wider adoption of intracameral antibiotic administration in the United States is the lack of a commercially available drug. Cefuroxime, for example, must be compounded manually, which exposes the patient to bacterial contamination and toxic anterior segment syndrome.
In lieu of cefuroxime, some American cataract surgeons are using intracameral administration of moxifloxacin and vancomycin to reduce or eliminate postoperative antibiotic drops.29 However, we must be cautious in recommending this practice––especially considering that, in the ESCRS study, patients still used levofloxacin postoperatively for one week.28
Newer-generation fluoroquinolones have become the topical antibiotic of choice in the prevention and treatment of ocular infections due to their high tissue concentrations, low MIC rates and broad spectrum of coverage. Nonetheless, eye care providers must be educated about the rising rates of increased fluoroquinolone resistance in certain pathogens, including MRSA.
We should make every effort to ensure an accurate diagnosis of bacterial infections to avoid unnecessary overuse of antibiotics. In cases where topical antibiotics are indicated, use them aggressively for a defined period. For infections that do not respond, perform cultures.
Today, we are fortunate to have several extremely effective antibiotic agents in our armamentarium. But, to prevail in the battle against ocular infection, we must use these drugs in a judicious capacity to limit further bactericidal resistance.
Dr. Muckley is the director of optometric services at Northeast Ohio Eye Surgeons in Kent and Stow, Ohio. She has no financial interest in any of the products mentioned. She can be contacted at 330-678-0201 or DrEDM1@aol.com.
1. Hooper DC. Fluoroquinolone antimicrobial agents. N Engl J Med. 1991 Feb 7;324(6):384-94.
2. Mah FS. Fourth-generation fluoroquinolones: New topical agents in the war on ocular bacterial infections. Curr Opin Ophthalmol. 2004 Aug;15(4):316–320.
3. Blondeau JM. Comparative in vitro activity of gatifloxacin, grepafloxacin, levofloxacin, moxifloxacin and trovafloxacin against 4151 gram-negative and gram-positive organisms. Int J Antimicrob Agents. 2000 Feb;14(1):45-50.
4. Strahilevitz J, Hooper DC. Dual targeting of topoisomerase IV and gyrase to reduce mutant selection: direct testing of the paradigm by using WCK-1734, a new fluoroquinolone, and ciprofloxacin. Antimicrob Agents Chemother. 2005 May;49(5):1949-56.
5. Dong Y. Fluoroquinolone action in mycobacteria: effects of C8 substitutions on bacterial growth, survival, and resistance. Antimicrob Agents Chemother. 1998 Nov;42(11):2978-2984.
6. Gardner S. Ocular drug penetration and pharmacokinetic principles. Clinical Ophthalmic Pharmacology. Boston, MA: Little Brown & Co;1987:1-52.
7. Holland EJ. Human ocular concentrations following topical fluoroquinolone adminstration relative to susceptibility of ocular pathogens. Invest Ophthalmol Vis Sci 2006;47: E-Abstract 1881.
8. Donnenfeld ED. Human aqueous humor concentrations of besifloxacin, moxifloxacin, and gatifloxacin after topical ocular application. J Cat Refract Surg. 2011Jun;37(6):1082-89.
9. McCaulley JP. Fourth generation fluoroquinolones penetration into aqueous humor in humans. Ophthalmology. 2006 June;113(6):955-59.
10. Woodcock J, Toigo T. Iquix. New Drug Application: Clinical Review. March 1, 2004. Available at:
www.accessdata.fda.gov/drugsatfda_docs/nda/2004/NDA%2021-571_Iquix_AdminCorres.pdf. Accessed November 17, 2012.
11. Lloyd RA. Zymaxid. New Drug Application: Clinical Review. July 30, 2009. Available at:
www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM214207.pdf. Accessed November 17, 2012.
12. Lindstrom R, Lane S, Cottingham A, et al. Conjunctival concentrations of a new ophthalmic solution formulation of moxifloxacin 0.5% in cataract surgery patients. J Ocul Pharmacol Ther. 2010 Dec;26(6):591-5.
13. Murphy MD, Mathis LL. Besivance. Executive Summary: Best Pharmaceuticals for Children Act Drug Use Review in the Pediatric Population. January 5, 2011. Available at:
www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM255069.pdf. Accessed November 17, 2012.
14. Ward KW, Lepage JF, Driot JY. Nonclinical pharmacodynamics, pharmacokinetics, and safety of BOL-303224-A, a novel fluoroquinolone antimicrobial agent for topical ophthalmic use. J Ocul Pharmacol Ther. 2007 Jun;23(3):243-56.
15. Noecker RJ, Herrygers LA, Anwaruddin R. Corneal and conjunctival changes caused by commonly used glaucoma medications. Cornea. 2004 Jul;23(5):490-6.
16. Okabe K, Kimura H, Okabe J, et al. Effect of benzalkonium chloride on transscleral drug delivery. Invest Ophthalmol Vis Sci. 2005 Feb;46(2):703-8.
17. Blondeau JM, Borsos S, Hesje CK. Antimicrobial efficacy of gatifloxacin and moxifloxacin with and without benzalkonium chloride compared with ciprofloxacin and levofloxacin against methicillin-resistant Staphylococcus aureus. J Chemother. 2007 Apr;19(2):146-51.
18. Friedlaender MH, Breshears D, Amoozgar B, et al. The dilution of benzalkonium chloride (BAK) in the tear film. Adv Ther. 2006 Nov-Dec;23(6):835-41.
19. Oliveira AD, D'Azevedo PA, Francisco W. In vitro activity of fluoroquinolones against ocular bacterial isolates in Sao Paulo, Brazil. Cornea. 2007 Feb;26(2):194-8.
20. Kowalski RP, Yates KA, Romanowski E, et al. An ophthalmologist's guide to understanding antibiotic susceptibility and minimum inhibitory concentration data. Ophthalmology. 2005 Nov;112(11):1987.
21. Gorwitz RJ. Understanding the success of methicillin-resistant Staphylococcus aureus strains causing epidemic disease in the community. J Infect Dis. 2008 Jan 15;197(2):179-82.
22. Haas W, Pillar CM, Torres M, et al. Monitoring antibiotic resistance in ocular microorganisms: Results from the Antibiotic Resistance Monitoring in Ocular micRorganisms (ARMOR) 2009 surveillance study. Am J Ophthalmol. 2011 Oct;152(4):567-574.e3.
23. Asbell PA, Sahm DF, Shaw M, et al. Increasing prevalence of methicillin resistance in serious ocular infections caused by Staphylococcus aureus in the United States: 2000 to 2005. J Cataract Refract Surg. 2008 May;34(5):814-8.
24. Asbell PA, Colby KA, Deng S. Ocular TRUST: nationwide antimicrobial susceptibility patterns in ocular isolates. Am J Ophthalmol. 2008 Jun;145(6):951-8.
25. Colleaux KM, Hamilton WK. Effect of prophylactic antibiotics and incision type on the incidence of endophthalmitis after cataract surgery. Can J Ophthalmol. 2000 Dec;35(7):373-8.
26. Moshirfar M, Feiz V, Vitale AT, et al. Endophthalmitis after uncomplicated cataract surgery with the use of fourth-generation fluoroquinolones: a retrospective observational case series. Ophthalmology. 2007 Apr;114(4):686-91.
27. Jensen MK, Fiscella RG, Moshirfar M, Mooney B. Third- and fourth-generation fluoroquinolones: retrospective comparison of endophthalmitis after cataract surgery performed over 10 years. J Cataract Refract Surg. 2008 Sep;34(9):1460-7.
28. Barry P, Seal DV, Gettinby G, et al. ESCRS study of prophylaxis of postoperative endophthalmitis after cataract surgery: Preliminary report of principal results from a European multicenter study. J Cataract Refract Surg. 2006 Mar;32(3):407-10.
29. Shorstein NH, Winthrop KL, Herrinton LJ. Decreased postoperative endophthalmitis rate after institution of intracameral antibiotics in a Northern California eye department. J Cataract Refract Surg. 2012 Oct 1. [Epub ahead of print]
30. Milder E. Changes in antibiotic resistance patterns of conjunctival flora due to repeated use of topical antibiotics after intravitral injection. Ophthalmology. 2012 Jul;119(7):1420-4.
31. Asbell PA. Ocular TRUST 3: Ongoing Longitudinal Surveillance of Antimicrobial Susceptibility in Ocular Isolates. Poster presented at: Annual Meeting of the American Society of Cataract and Refractive Surgery: April 3-8, 2009; San Francisco.
32. Bababeygy SR, Silva RA, Sun Y, Jain A. Rifampin and linezolid in the treatment of methicillin-resistant Staphylococcus aureus preseptal cellulitis. Ophthal Plast Reconstr Surg. 2009 May-Jun;25(3):227-8.
33. Hoffmann CJ. Mycobacterium chelonae. Johns Hopkins Antibiotic (ABX) Guide. Available at:
www.hopkinsguides.com/hopkins/ub/view/Johns_Hopkins_ABX_Guide/540364/all/Mycobacterium+chelonae. Accessed November 17, 2012.

