Cornea ReportCheck out the other feature articles in this month's issue: |
Many systemic diseases have ocular manifestations, and it is common for patients to present to their eye doctor with ocular symptoms. Optometrists are often the first to encounter these patients and play a significant role in identifying and managing ocular manifestations of systemic diseases. Eye care providers must work closely with the appropriate medical provider to improve outcomes. In this review, we will describe the clinical presentation, etiology and management of four systemic diseases—Sjögren’s syndrome (SS), atopic disease, graft-vs.-host disease (GVHD) and facial nerve palsy secondary to a variety of systemic etiologies.
Sjögren’s Syndrome
This is a chronic autoimmune disease that affects the exocrine glands of mucus membranes, including the lacrimal and salivary glands.1 SS is the second most common rheumatologic disease, commonly affecting woman in their fourth or fifth decade.2 Secondary disease is associated with autoimmune conditions such as rheumatoid arthritis, systemic lupus erythematousus and Wegner’s granulomatosis.3 Lymphocytic infiltration and inflammation of the lacrimal gland leads to decreased production of tears.1 The reduction in aqueous secretion leads to dryness of the ocular surface and a reduced ability to remove debris, waste and microbes, which normally drain with the tears.
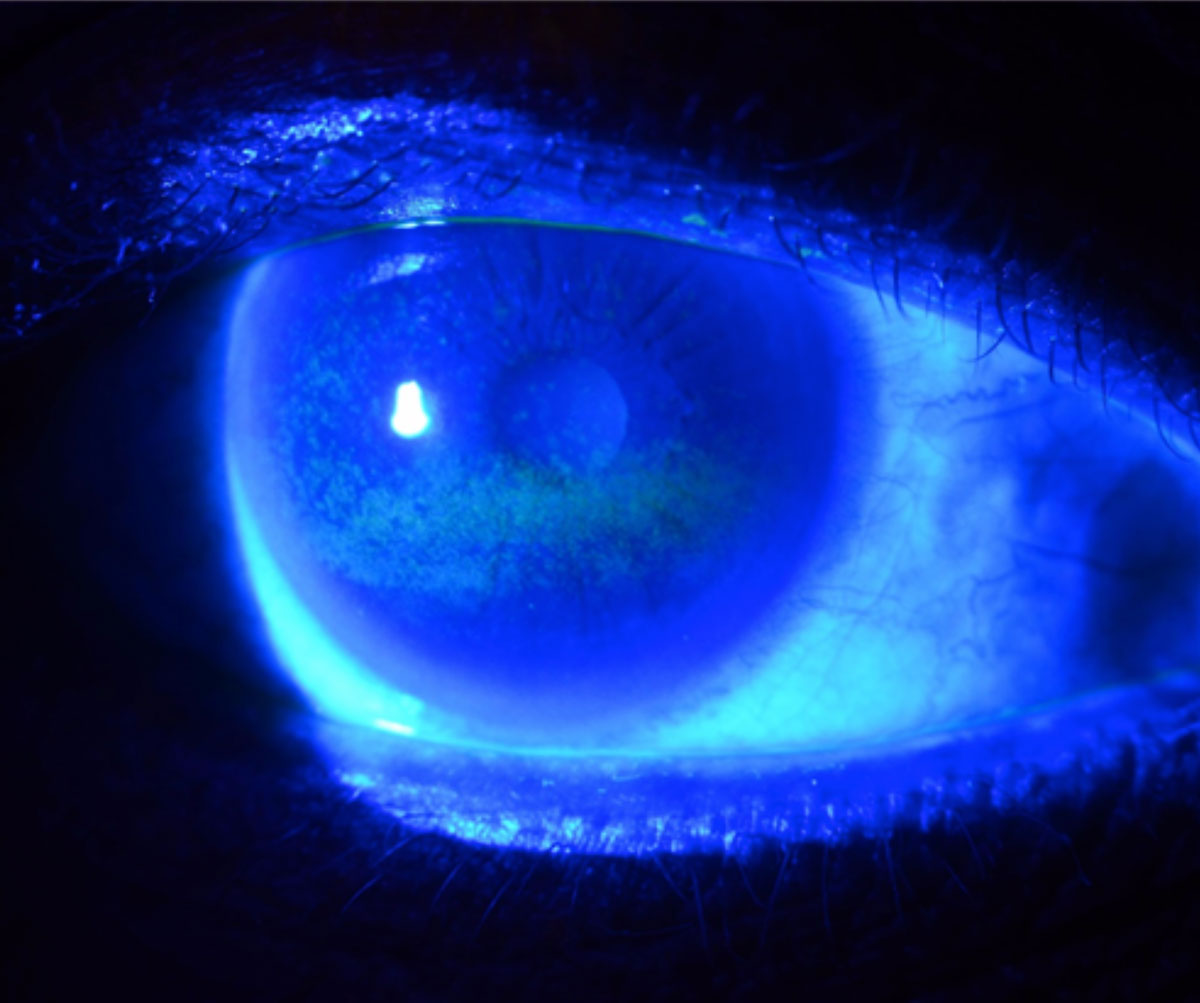 |
| Fig. 1. This is a typical fluorescein staining pattern in a patient with severe dry eye from Sjögren’s syndrome. Photo: WR Buie, ophthalmic photographer. Click image to enlarge. |
Keratoconjunctivitis sicca (KCS), or dry eye disease, is a key finding in SS and in many inflammatory, autoimmune and collagen-vascular disorders. KCS is a multifactorial disease that involves changes in the tear film, resulting in damage to the ocular surface.4 SS-related dry eye is associated with aqueous deficiency, although these patients may also have evaporative tear loss. Corneal manifestations range from punctate epithelial erosions to severe sight-threatening complications such as ulcers, perforation and scarring (Figures 1 and 2). Corneal ulcers associated with SS are sterile, usually located centrally and are circular or oval in shape and less than 3mm in size.2 These require prompt referral to a cornea specialist to prevent irreversible vision loss.
Evaluation of patients with SS requires a thorough history and clinical examination of the cornea, conjunctiva and eyelids. Clinicians should stain the ocular surface with fluorescein and assess tear film break-up time (TBUT) to evaluate the tear film stability and epithelial integrity. Fluorescein staining of the epithelium indicates increased epithelial permeability.5 The tear film exhibits reduced volume and quality in KCS, and a thin tear meniscus is suggestive of aqueous deficiency.6 The Shirmer test, which can quantify aqueous deficiency and lacrimal function, will often yield a result of less than 5mm in five minutes in patients with SS.5
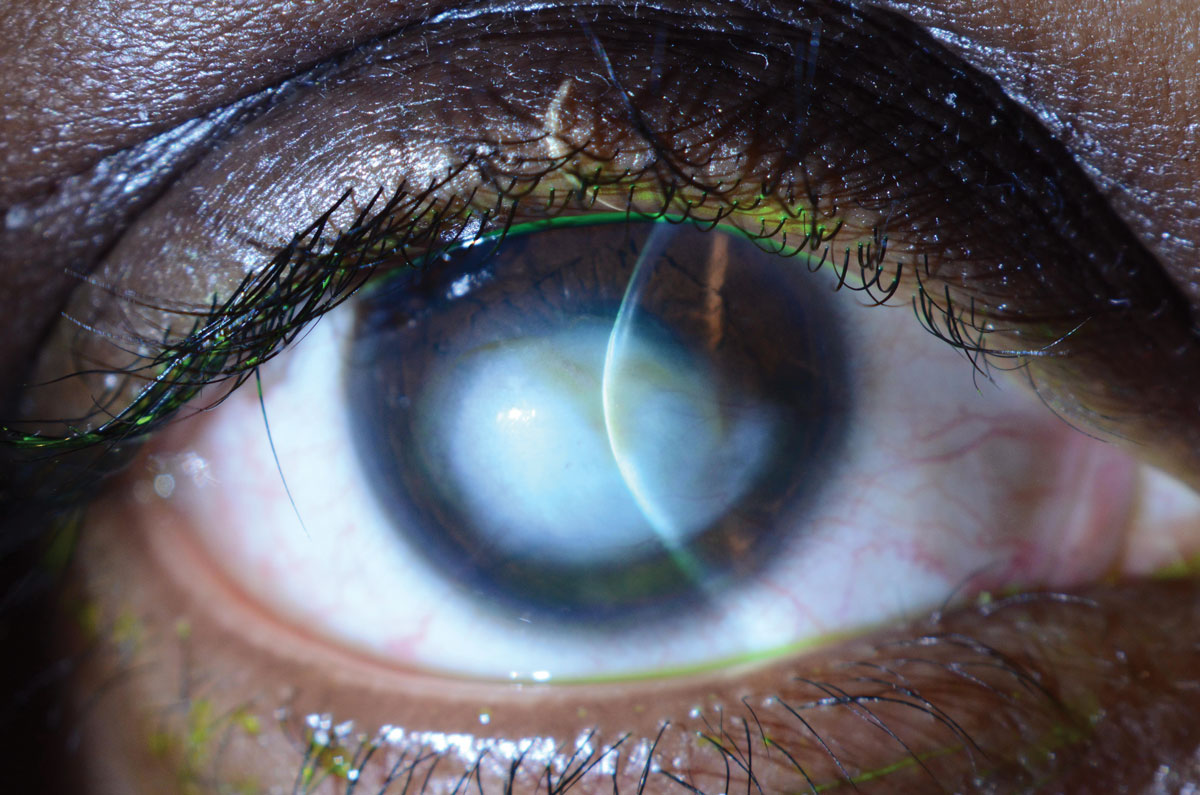 |
| Fig. 2. This patient with Sjögren’s syndrome has a central corneal scar following corneal perforation. Photo: WR Buie, ophthalmic photographer. Click image to enlarge. |
The goals of managing patients with SS include treating symptoms of KCS and preventing sight-threatening corneal complications. The first-line treatment is lubrication of the ocular surface with artificial tears, gels and ointment. Tear preservation with punctal occlusion is useful in patients with aqueous deficiency and staining of the ocular surface.7 Ocular surface inflammation should be treated prior to insertion of punctal plugs. Anti-inflammatory therapy may include topical cyclosporine, steroids and oral tetracycline.8 Lifitegrast is a new small-molecule integrin antagonist that is useful in the treatment of KCS. The essential fatty acids, omega-3s, have both anti-inflammatory properties as well as efficacy in preventing tear evaporation by improving tear quality.9 Research shows a diet rich in essential fatty acids or nutritional supplementation improves patient symptoms based on the Ocular Surface Disease Index score.10 Sources of omega-3 essential fatty acids include cold-water fish, such as tuna and salmon, as well as flaxseed, chia seed and green leafy vegetables.
Atopic Disease
Allergic eye disease can be categorized as atopic keratoconjunctivitis, vernal keratoconjunctivitis or seasonal keratoconjunctivitis.
Atopic keratoconjunctivitis (AKC) is associated with systemic allergic diseases such as environmental allergies, asthma and eczema. It is a chronic type IV hypersensitivity immune inflammatory response with episodes of exacerbation and remission. Chronic AKC can affect the eyelids, conjunctiva, cornea or lens.
Eyelid complications of AKC include periorbital atopic dermatitis, blepharitis, lid thickening, scarring and maderosis, ectropion or entropion.11 Conjunctival manifestations of ACK include papillae on the tarsal conjunctiva, cicatricial changes and symblepharon formation. Anterior subcapsular cataracts are associated with atopic disease, and posterior subcapsular cataracts can result from chronic steroid use.
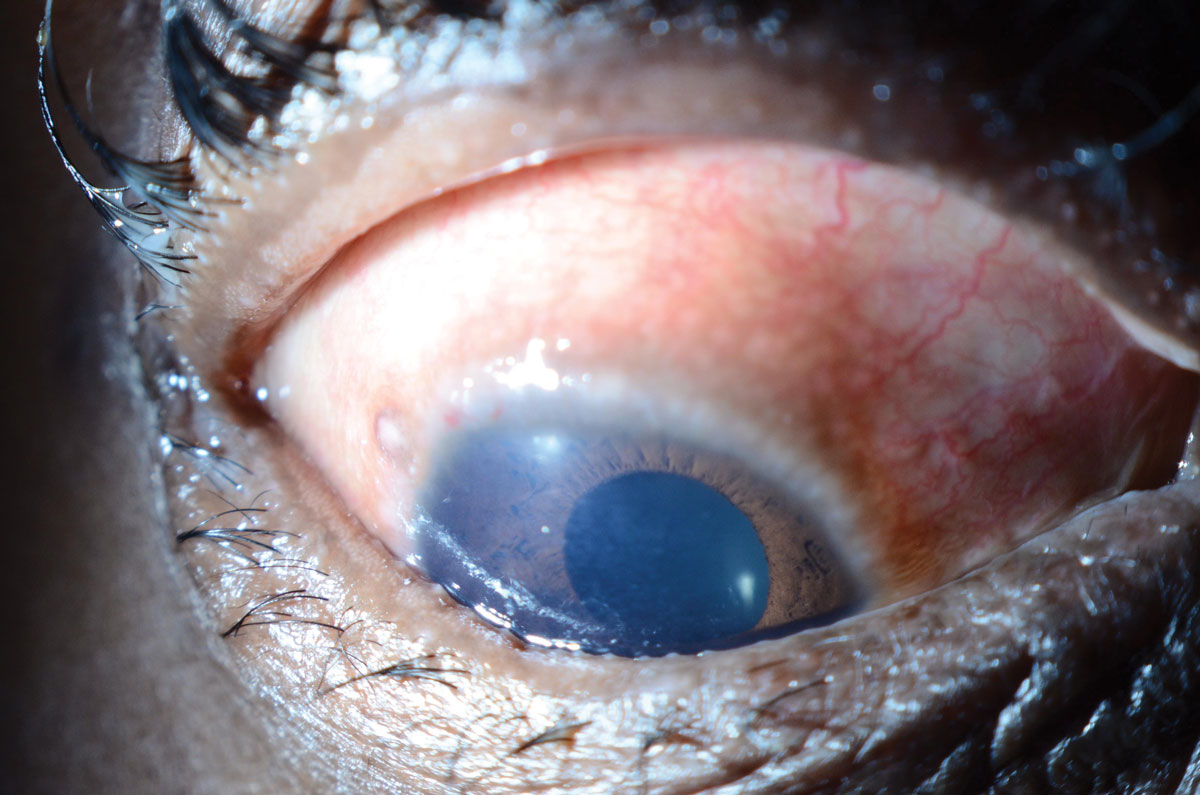 |
| Fig. 3. This patient with vernal keratoconjunctivitis presented with limbal Horner-Trantas Dots. Photo: WR Buie, ophthalmic photographer. Click image to enlarge. |
Corneal manifestations of AKC include superficial punctate keratopathy, filaments and neovascularization. Trauma from repeated eye rubbing or inflamed conjunctiva can result in corneal erosions, scarring and corneal ectasias such as keratoconus.11 Corneal infections with Staphylococcus aureus or herpes simplex virus are more common in patients with AKC, as are squamous neoplasia of the conjunctiva or cornea.
Seasonal keratoconjunctivitis is caused by an allergic response to allergens such as grass, pollen, animal dander or dust and can recur with seasonal changes. This is an IgE-mediated response with release of mast cells and inflammatory markers. Symptoms include conjunctival injection, watery or mucoid discharge and chemosis.
Vernal keratoconjunctivitis occurs in response to environmental allergens and is worse during the spring.12 It is more common in males with a peak incidence in childhood and typically resolves in early adulthood. Ocular signs of vernal keratoconjunctivitis include cobblestoning of the conjunctiva, corneal neovascularization and limbal Horner-Trantas dots (Figure 3). Corneal complications of vernal keratoconjunctivitis can includes progression to shield ulcers (Figure 4).
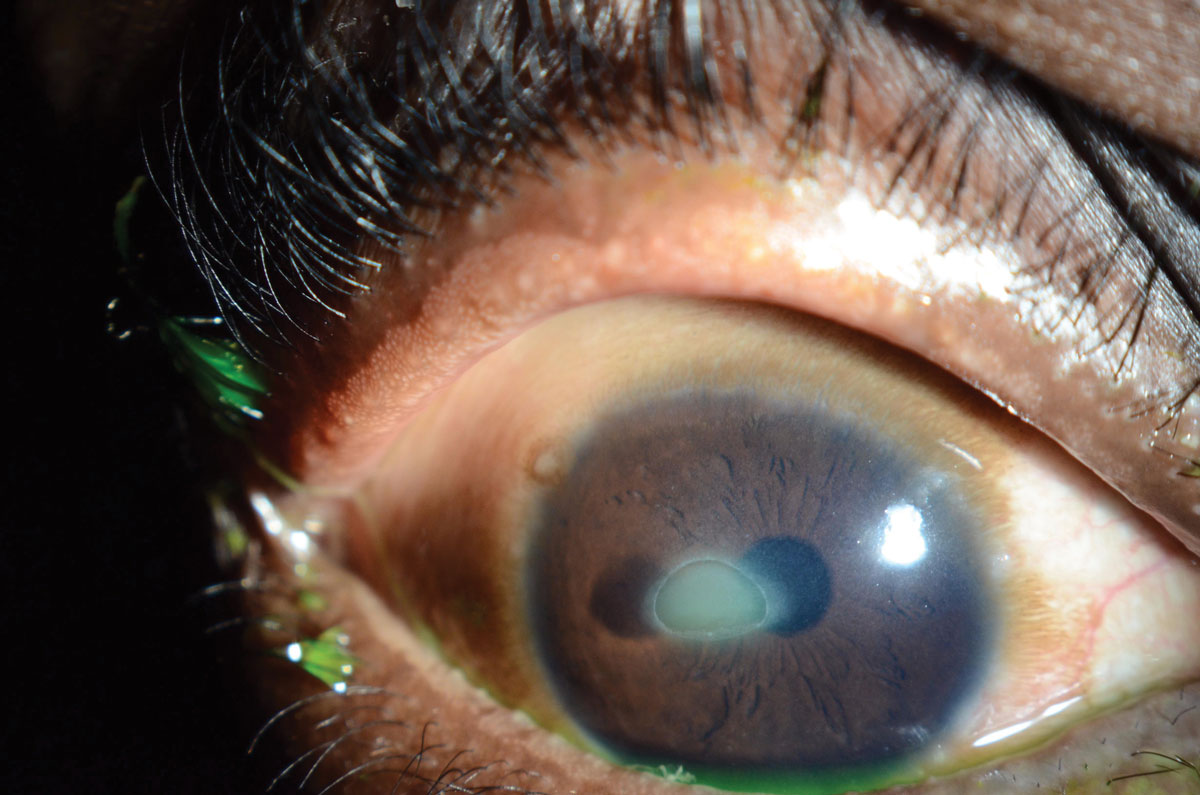 |
| Fig. 4. Shield ulcers are possible in patients with vernal keratoconjunctivitis. Photo: WR Buie, ophthalmic photographer. Click image to enlarge. |
Management of allergic eye diseases, including seasonal, atopic and vernal keratoconjunctivitis, should focus on treating both chronic symptoms and acute flares. Patients should avoid allergens and employ supportive care such as cool compresses and ocular lubrication to improve symptoms. Artificial tears not only lubricate the ocular surface, but can dilute pathogens and decrease itching. Topical H-1 receptor antagonists, mast-cell stabilizers or combination drops can control itching in seasonal and vernal keratoconjunctivits, but are less effective in AKC. For severe AKC, topical treatment with cyclosporine drops or tacrolimus is warranted. Topical corticosteroids may be indicated for exacerbations but are not recommended for long-term use due to side effects. Vasoconstrictor drops should be avoided in allergic eye disease due to rebound hyperemia.
Graft-vs.-host Disease
GVHD is a collection of complications occurring after allogeneic hematopoietic stem cell transplantation, which is performed for a variety of benign or malignant hematological diseases, inherited metabolic disorders and autoimmune diseases.13 During the procedure, stem cells are harvested from bone marrow, peripheral blood or placental blood from donors who are human leukocyte antigen matched.13-16 Alloreactivity occurs between the donor lymphocytes and host tissue to destroy the tumor, but also attacks non-malignant cells, which results in GVHD.17
This condition occurs primarily in the skin, liver and gastrointestinal system and may also involve the lungs, esophagus and oral and ocular mucosa.13,18-20 Risk factors for both acute and chronic GVHD include female donor to male recipient, unrelated donor, older-aged recipient and stem cells extracted from the peripheral blood.
Ocular GVHD occurs in approximately 40% to 60% of patients receiving allogeneic hematopoietic stem cell transplantation, and it is considered a poor prognostic factor for mortality.20-24 Conjunctival hyperemia in the setting of systemic GVHD is highly suggestive of acute ocular GVHD.25 The occurrence and severity of acute ocular GVHD correlates with the severity of the systemic disease.13
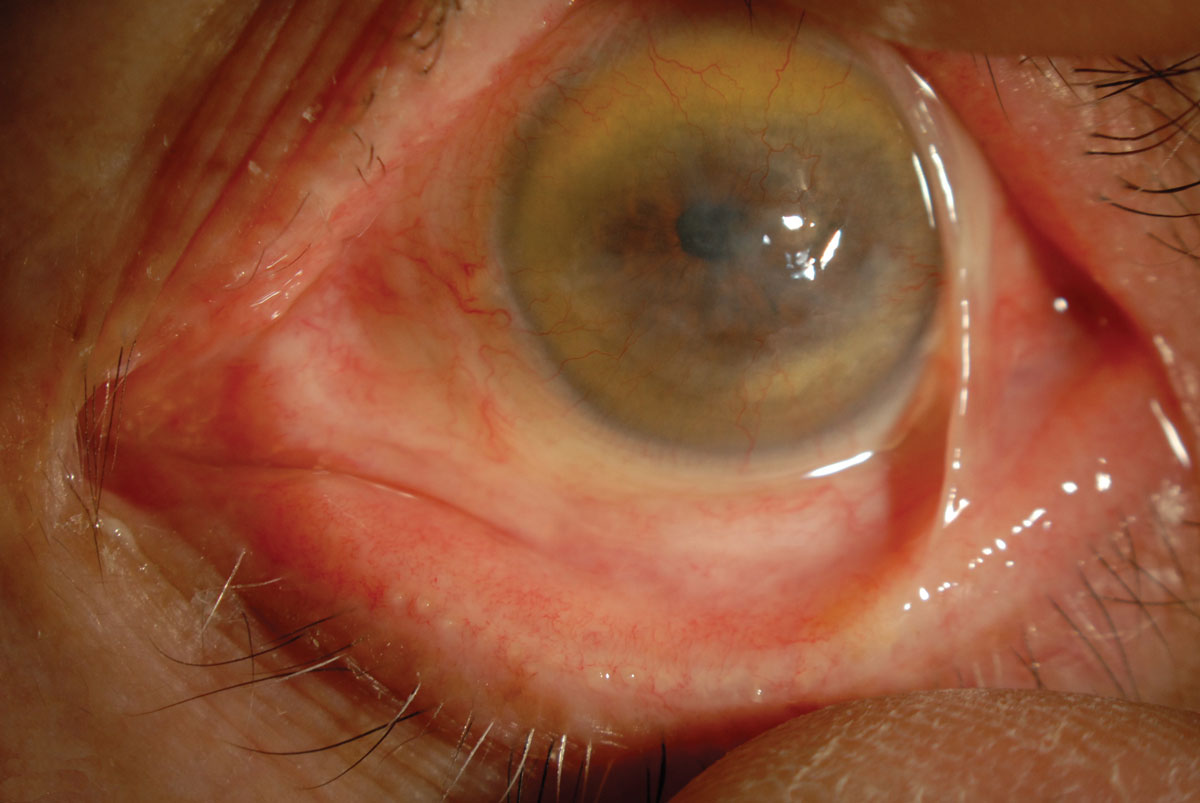 |
| Fig. 5. Patients with severe ocular GVHD may present with symblepharon and corneal neovascularization. Photo: W. Munir, MD. Click image to enlarge. |
This multifactorial disease primarily manifests as KCS and also involves destruction and fibrosis of the conjunctiva and lacrimal gland. This may lead to decreased aqueous tear production along with meibomian gland dysfunction.14 Conjunctival involvement may present in varying degrees, from mild hyperemia to pseudomembranous conjunctivitis to cicatricial changes with symblepharon formation (Figure 5).
Corneal manifestations of ocular GVHD are similar to those of KCS, including punctate epithelial keratopathy. Patients may present with painful epithelial erosions and filamentary keratitis. Additional corneal complications include limbal stem cell deficiency, non-healing ulcers, stromal thinning and necrosis, which may progress to corneal perforation. Less frequent ocular manifestations of GVHD may include uveitis, scleritis, retinal complications and cataract and glaucoma due to prolonged steroid use.13,26
When evaluating patients who have undergone hematopoetic stem cell transplantation, clinicians must obtain a thorough medical and ocular history, perform a comprehensive eye exam at baseline and repeat the exam 100 to 200 days after transplantation. TBUT with fluorescein dye and Schirmer test without anesthesia are useful tools for this population.
In treating the corneal manifestations, the same systematic approach should be taken to manage symptoms of KCS and prevent sight-threatening complications. Filamentary keratitis requires careful removal of filaments. Epithelial support with autologous serum tears or amniotic membrane may help treat symptoms and prevent worsening of corneal disease.25 Scleral contact lenses may help prevent complications by providing continuous hydration to the corneal surface.27 These treatments may prevent surgical intervention.
Comanagement with a cornea specialist is recommended in severe cases such as non-healing epithelial defects or risk of perforation, which may require tarsorrhaphy, limbal cell transplantation, fornix reconstruction or corneal transplantation.27
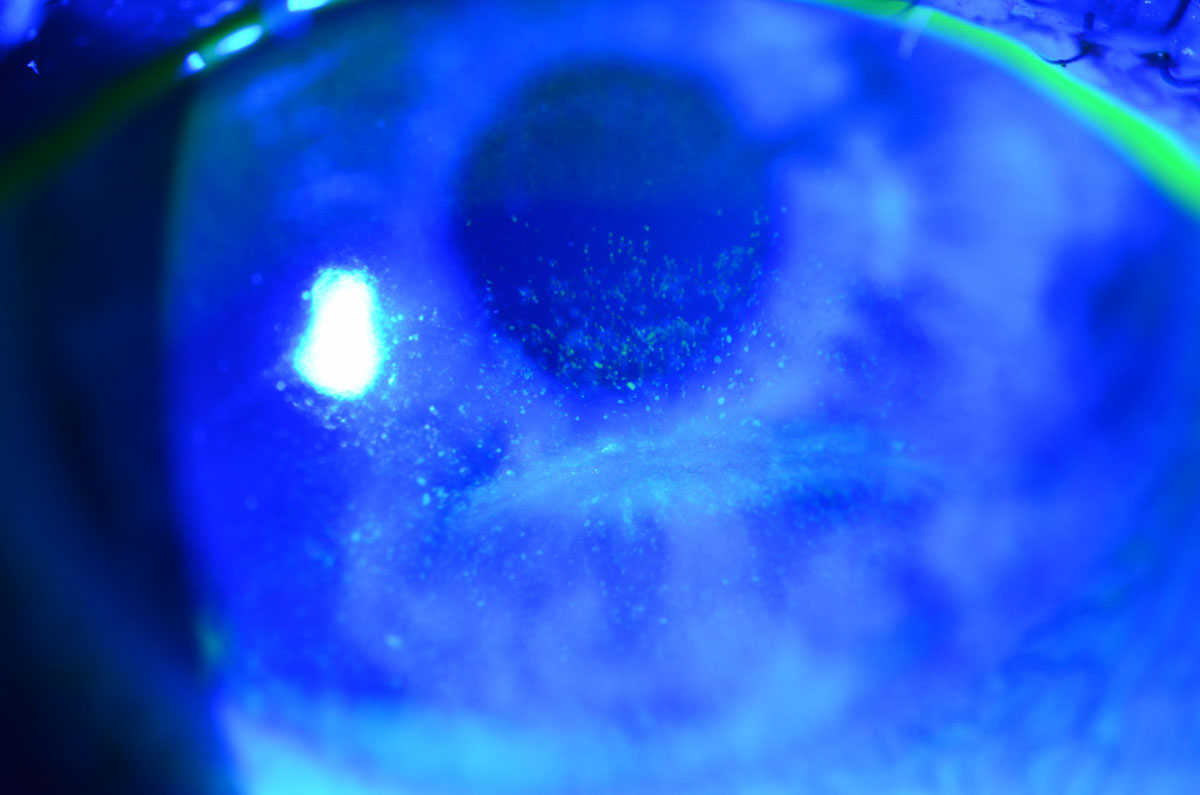 |
| Fig. 6. This patient with lagophthalmos from facial nerve paralysis has interpalpebral corneal staining. Photo: WR Buie, ophthalmic photographer. Click image to enlarge. |
Facial Nerve Palsy
Many systemic diseases can lead to facial nerve palsy, including infection, tumor and stroke. Facial palsy may also result from trauma, surgery or may be idiopathic (Bell’s palsy). Bell’s palsy is the most common etiology, although evidence suggests that herpes simplex may be the cause of facial nerve palsies thought to be idiopathic.28,29 Of patients with Bell’s palsy, 83% have a good recovery.30 Ramsay-Hunt syndrome, which results from herpes zoster infection, is associated with painful paralysis and is less likely to have complete recovery than Bell’s palsy.30
Optometrists frequently care for patients with facial nerve palsy and treat resulting symptoms of epiphora and dry eye. Patients may present with these symptoms acutely, or may be referred from another medical specialist treating them for tumor, infection or trauma.
The most common corneal manifestation of facial nerve palsy is corneal exposure, which can range in severity from mild dryness to corneal ulceration and blindness. Exposure results from lack of corneal protection when lagophthalmos is present. Facial nerve palsy causes loss of orbicularis function and blink response. Lack of Bell’s reflex and reduced corneal sensation increase the risk of corneal injury from lagophthalmos.
These patients require a comprehensive eye examination that specifically focuses on the eyelids and cornea. When evaluating eyelids, clinicians should look for lid-globe congruity, lower eyelid retraction and symmetry of the palpebral fissure between the two eyes. In addition, ensure the puncta is patent and check whether it is everted or rotated inwards. This should be evaluated during active blinking to also check for incomplete eyelid closure.31
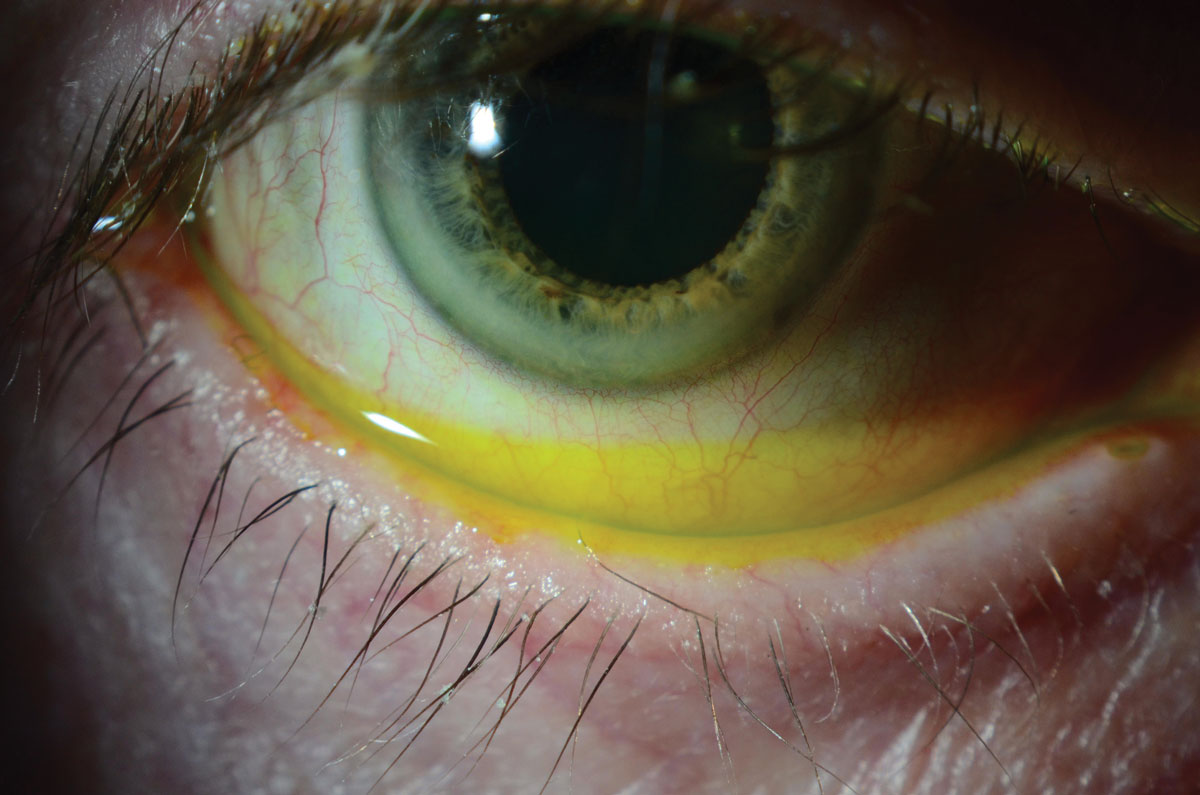 |
| Fig. 7. This evaluation of the tear film with fluorescein reveals a high tear lake in a patient with paralytic lagophthalmos following surgery for squamous cell carcinoma of the parotid gland. Photo: WR Buie, ophthalmic photographer. Click image to enlarge. |
The corneal evaluation includes identifying areas of dryness on the unprotected corneal surface (Figure 6). Clinicians should check the tear meniscus for thickness and quality of tears, including whether there is debris or foamy build-up. Clinicians can evaluate the tear meniscus by adding fluorescein, but too much fluid will increase the tear lake (Figure 7).
The main treatment goal for patients with facial nerve palsy is to protect the cornea and preserve good visual acuity. This requires a multidisciplinary approach, often encompassing otolaryngology surgeons and oculoplastics specialists.28 The optometrist’s role is to protect the ocular surface, treat any exposure keratopathy and prevent complications, such as corneal perforation, by implementing techniques described above, as well as by offering solutions for temporary lid closure such as adhesive tapes or masks.
Surgical management by an oculoplastics specialist is indicated when supportive therapy inadequately controls symptoms or in worsening of corneal disease. Tarsorrhaphy is indicated when incomplete eyelid closure occurs in combination with reduced corneal sensation, as this combination increases risk of corneal perforation.32 Botulinum toxin injections can be used to temporarily paralyze the levator muscle, inducing a ptosis that protects the cornea and may prevent the need for tarsorrhaphy is some patients.32 Gold or platinum weight implantation is an effective treatment for patients with paralytic lagophthalmos.33 Once the risk of corneal ulceration is eliminated, patients may undergo brow ptosis repair or blepharoplasty for improved cosmesis.
These are just a few of many systemic diseases that affect the cornea. Optometrists must work with other sub-specialists to manage the disease, control ocular symptoms and prevent serious visually threatening complications.
Drs.Wilkins, Munir and Levin are faculty at the University of Maryland School of Medicine in the Department of Ophthalmology and Visual Sciences.
Treatment Summaries for Corneal Manifestations of Systemic Diseases | ||
| Disease | Etiology | Corneal Treatment |
| Sjögren’s Syndrome |
| Treatment of keratoconjunctivitis sicca:
|
| Atopy |
|
|
| Ocular GVHD |
|
|
| Facial Nerve Palsy |
|
|
|
1. Mavragani CP, Moutsopoulos HM. Sjögren syndrome. CMAJ. 2014;186(15):E579-86. 2. Nassiri N, Djalilian A, Hamrah P, Pglugfelder S. Dry Eye. In: Krachmer JH, Mannis MJ, Holland EJ, eds. Cornea. St. Louis: Mosby/Elsevier; 2011:919-44. 3. Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;1:554-8. 4. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007;5:75-92. 5. Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007;5:108-52. 6. GN Foulks GN. Clinical guidelines for management of dry eye associated with Sjögren disease. Ocul Surf. 2015;13(2):118-32. 7. Baxter SA, Laibson PR. Punctal plugs in the management of dry eyes. Ocul Surf. 2004;2:255-65. 8. Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007;5:163-78. 9. Barabino S, Horwath-Winter J, Messmer EM, et al. The role of systemic and topical fatty acids for dry eye treatment. Prog Retin Eye Res. 2017 Nov;61:23-34. 10. The Dry Eye Assessment and Management Study Research Group. n−3 fatty acid supplementation for the treatment of dry eye disease. N Engl J Med. 2018;378:1681-90. 11. Patel N, Venkateswaran N, Wang Z, Galor A. Ocular involvement in atopic disease: a review. Curr Opin Ophthalmol. 2018;29(6):576-81. 12. Andalibi S, Haidara M, Bor N, Levin M. An update on neonatal and pediatric conjunctivitis. Current Ophthalmology Reports. 2015;3(3):158-69. 13. Munir SZ, Aylward J. A review of ocular graft-versus-host disease. Optom Vis Sci.2017;94:545-55. 14. Hessen M, Akpek EK. Ocular graft-versus-host disease: Curr Opin Allergy Clin Immunol. 2012;12:540-7. 15. Espana EM, Shah S, Santhiago MR, Singh AD. Graft versus host disease: clinical evaluation, diagnosis and management. Graefes Arch Clin Exp Ophthalmol. 2013;251:1257-66. 16. Nassar A, Tabbara KF, Aljurf M. Ocular manifestations of graft-versus-host disease. Saudi J Ophthalmol. 2013;27:215-22. 17. von Bonin M, Bornhäuser M. Concise review: the bone marrow niche as a target of graft versus host disease. Stem Cells. 2014;32:1420-8. 18. Sung AD, Chao NJ. Acute graft-versus-host disease: Are we close to bringing the bench to the bedside? Best Pract Res Clin Haematol. 2013;26:285-92. 19. Magenau J, Runaas L, Reddy P. Advances in understanding the pathogenesis of graft-versus-host disease. Br J Haematol. 2016;173:190-205. 20. Qian L, Wu Z, Shen J. Advances in the treatment of acute graft-versus-host disease. J Cell Mol Med. 2013;17:966-75. 21. Ogawa Y, Kim SK, Dana R, et al; International Chronic Ocular Graft-vs-Host-Disease (GVHD) Consensus Group: proposed diagnostic criteria for chronic GVHD (Part I). Sci Rep. 2013;3:3419. 22. Na K-S, Yoo Y-S, Mok JW, et al. Incidence and risk factors for ocular GVHD after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2015;50:1459-64. 23. Wang JCC, Teichman JC, Mustafa M, et al. Risk factors for the development of ocular graft-versus-host disease (GVHD) dry eye syndrome in patients with chronic GVHD. Br J Ophthalmol. 2015;99:1514-18. 24. Townley JR, Dana R, Jacobs DS. Keratoconjunctivitis sicca manifestations in ocular graft versus host disease: pathogenesis, presentation, prevention, and treatment. Semin Ophthalmol. 2011;26:251-60. 25. Shikari H, Antin JH, Dana R. Ocular graft-versus-host disease: a review. Surv Ophthalmol. 2013;58:233-51. 26. Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant J. 2015;21:389-401.e1. 27. Harthan JS, Shorter E. Therapeutic uses of scleral contact lenses for ocular surface disease: patient selection and special considerations. Clin Optom (Auckl). 2018;10:65-74. 28. Rahman I, Sadiq SA. Ophthalmic management of facial nerve palsy: a review. Surv Ophthalmol. 2007;52(2):121-44. 29. Musani MA, Farooqui AN, Usman A, et al. Association of herpes simplex virus infection and Bell’s palsy. J Pak Med Assoc. 2009;59(12):823-5. 30. Peitersen E. Bell’s palsy: the spontaneous course in 2500 peripheral facial nerve palsies of different aetiologies. Acta Otolaryngol. 2002;549(Suppl):4-30. 31. Lane C. Management of ocular surface exposure. Br J Ophthalmol. 2012;96:471-72. 32. Lee V, Currie Z, Collin JR. Ophthalmic management of facial nerve palsy. Eye (Lond). 2004;18(12):1225-34. 33. Yu Y, Sun J, Chen L, Liu L. Lid loading for treatment of paralytic lagophthalmos. Aesth Plast Surg. 2011;35:1165. |

