A Hollenhorst plaque is a relatively common retinal finding in the geriatric population. Patients often are visually asymptomatic and present with retinal emboli from plaque ulceration in the internal carotid artery. Carotid duplex is a high-quality, first-line test that identifies the degree of lumen narrowing. Recommendations for medical therapy or surgical intervention are largely based on the amount of stenosis, and may be further guided by diagnostic imaging modalities.
Here, we review the case of a 62-year-old white male who presented with multiple Hollenhorst plaques, a twig retinal artery occlusion and hemodynamically significant internal carotid stenosis.
History
A 62-year-old white male presented to Harry S. Truman Memorial Veterans’ Hospital optometry clinic in Columbia, Mo., for a
routine diabetic eye exam on March 22, 2011. The patient’s only complaint was visually distracting scratches on his glasses.
His systemic history was remarkable for lower back pain, hypertension, hypercholesterolemia and a 10-year history of type 2 diabetes mellitus. His current medications included 50mcg fluticasone nasal spray, 600mg gemfibrozil, 20mg glipizide, 12.5mg hydrochlorothiazide, 80mg lisinopril, 2,000mg metformin hcl, 1,000mg naproxen, 30mg pioglitazone and 30mg tramadol hcl.
At a routine check-up with his primary care physician one month earlier, his blood pressure measured 157/71mm Hg. His most recent hemoglobin A1C measurement was 6.8% from a previous primary care appointment in November of 2010.
At his last ocular evaluation one year earlier, the patient exhibited incipient cataracts, mild dermatochalasis, compound hyperopic astigmatism and an unremarkable dilated fundus examination. His family history was unremarkable for known ocular disorders.
Diagnostic Data
Best-corrected visual acuity was 20/20 OU. Extraocular motility was full and unrestricted in both eyes. Confrontation fields were unremarkable OD; however, we noted a small scotoma located inferior to fixation OS. The defect was repeatable on Amsler grid testing.
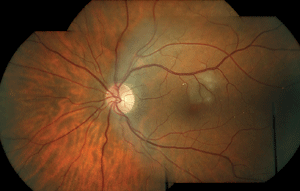
1. Color fundus photo montage of our patient’s left eye at initial presentation on March 22, 2011. We noted multiple Hollenhorst plaques located throughout the temporal arcades. Further, we documented a twig retinal artery occlusion located superior to the fovea.
Pupils were equal, round and reactive to light, without evidence of afferent defect OU. Slit-lamp exam was remarkable for mild dermatochalasis. The anterior chamber was deep and quiet in both eyes. Intraocular pressure measured 17mm Hg OD and 15mm Hg OS. Dilated slit-lamp examination revealed trace nuclear sclerotic lenticular opacities in both eyes.
Funduscopy was performed with a 78D lens and showed a 0.15 x 0.15 cup-to-disc ratio, with healthy neuroretinal rim tissue in both eyes. The right fundus was unremarkable except for a resolving retinal microinfarct located along the superotemporal arcade.
The left eye exhibited 13 separate retinal emboli lodged in the superior and inferior temporal retinal arterioles (figure 1). Many of the retinal emboli were refractile and located at arterial bifurcations. We also documented a 1DD area of white, mildly edematous retina located superior to the fovea. There was no evidence of vitreal or retinal inflammation. The peripheral retina was flat and intact bilaterally. Carotid auscultation did not reveal a bruit on either internal carotid artery.
The patient was referred for a lipid panel, carotid duplex and vascular surgery consult, given the high suspicion for hemodynamically significant stenosis in the left internal carotid artery.
Differential Diagnoses
The differential diagnoses for retinal arterial emboli in this case included:
• Calcific emboli. Nonscintillating, white in appearance, and typically present in the central retinal artery due to their large size. Such entities may remain in the retinal vasculature permanently, because they do not dissolve. They are associated with heart valve or aorta calcification. Transesophageal echocardiogram is needed to confirm the diagnosis.
• Fibrinoplatelet emboli. Dull white in appearance and often present as long, smooth emboli simulating a plug in the retinal arteriole. They are most commonly associated with carotid thrombosis.
• Cholesterol emboli/Hollenhorst plaque. Highly refractile, crystal-like emboli that typically are seen at arteriole bifurcations. The emboli can be visually asymptomatic, because they often do not cause a significant obstruction of the retinal arteriole. Also known as Hollenhorst plaques, cholesterol emboli originate from atheromatous lesions in the ipsilateral carotid artery or aorta.
• Talc emboli. Associated with intravenous drug injection and free-base cocaine use. The talc particles are small and white, and frequently found parafoveally.
• Tumor cells. Commonly caused by metastatic lesions, these proliferative neoplastic cells may separate from the lesion and lodge in the retinal arterioles.
• Septic emboli. These deposits are associated with bacterial endocarditis.
• Fat emboli. An uncommon cause of emboli due to long bone fractures. They are associated with Purtcher’s retinopathy. Concomitant scattered retinal microinfarcts and hemorrhages typically are associated with the condition.
Diagnosis
We diagnosed the patient with a twig retinal artery occlusion secondary to Hollenhorst plaques. On initial examination, the emboli appeared refractile and were located in multiple arterial bifurcations. Although multiple types of emboli can present in the retinal vasculature system, the findings were most consistent with atheromatous changes in the carotid artery. If carotid duplex results were non-contributory, additional testing would have been conducted.
Follow-up and Treatment
• Follow up #1. A local vascular surgeon evaluated the patient approximately three weeks after the initial visit. A carotid duplex was performed, which revealed no significant stenosis in the right carotid artery system. The left internal carotid artery had a peak systolic velocity of 247cm/sec, an end diastolic velocity of 81cm/sec and greater than 70% stenosis. The surgeon recommended a carotid endarterectomy (CEA), to which the patient consented.
He underwent a CEA of the left internal carotid artery on April 21, 2011. The procedure was performed without perioperative complications. A repeat carotid duplex two weeks later revealed a clear and patent internal carotid artery. Carotid duplex imaging was recommended at one-year follow up.
• Follow up #2. The patient returned to the eye clinic on June 22, 2011 for bilateral dilation. Visual acuity remained 20/20 in each eye with spectacle correction. Once again, he denied experiencing any visual complaints. Further, he suggested that he felt better since undergoing the CEA.
All entrance testing and anterior segment findings were unchanged. Dilated fundus examination was unremarkable OD. The left fundus, however, exhibited a retinal microinfarct located along the superotemporal arcade as well as an isolated Hollenhorst plaque located nasal to the optic nerve head (figure 2). There was no evidence of plaques lodged in the temporal retinal arcades.
• Follow up #3. He returned on December 19, 2011 for bilateral dilation. Visual acuity was 20/20-2 OU with spectacle correction. The patient again denied visual complaints since undergoing the CEA eight months earlier.
All entrance testing and anterior segment findings remained unchanged from the initial exam. The fundus was unremarkable in both eyes. No hemorrhages, microinfarcts or emboli were detected in either eye.
Discussion
Ophthalmologist Robert W. Hollenhorst first described cholesterol emboli in the retinal arterioles in 1958.1 A Hollenhorst plaque is an embolus formed from cholesterol deposition that typically originates from the ipsilateral carotid artery. They appear as refractile, crystal-like emboli and usually are lodged at arteriole bifurcations. Patients are largely asymptomatic due to plaque malleability as well as persistent vascular perfusion around the emboli.
Hollenhorst plaques often are discovered incidentally during funduscopy. Frequently, the plaques dislodge and are not noted on subsequent examinations.2 Patients may experience amaurosis fugax if the plaque becomes lodged in a retinal arteriole for a transient period. Cases also can present with simultaneous evidence of a retinal artery occlusion and corresponding visual scotomas. Retinal artery occlusions occur if the embolus size is larger than the caliber of the vessel it enters––thereby preventing perfusion to a specific portion of the retina. Retinal ischemia results distal to the arteriole blockage.2
Many patients with cholesterol emboli are of geriatric age and have comorbid vascular conditions. The occurrence of Hollenhorst plaques and retinal artery occlusions has long concerned clinicians because of its potential neurologic involvement. A carotid duplex is the primary modality of carotid artery imaging, because it is a non-invasive procedure. Either CEA or carotid angioplasty and stenting (CAS) typically are performed in patients with hemodynamically significant stenosis.
• Pathophysiology. Atherosclerosis is a form of arteriosclerosis, during which the intimal layer of the vascular column in both medium and large arteries hardens secondary to progressive plaque formation.
Although the exact mechanism of atherosclerosis remains unknown, clinicians generally agree that hypertension, hyperlipidemia, diabetes and cigarette smoking are associated risk factors. Further, diets rich in cholesterol (particularly low-density lipoproteins) appear to alter the permeability of the arterial wall endothelium. Circulating monocytes are then able to adhere to the compromised surface, allowing the passage of lipoproteins through active transport.
Lipoproteins filter through the basement membrane and simultaneously draw the monocytes through the endothelial wall into the intimal layer. The monocyte converts into a macrophage and phagocytizes the lipoproteins to form a “foam cell.” Foam cells accumulate in the tunica intima and create a roughened/irregular endothelial surface that facilitates platelet aggregation.
Biochemical signals for fibrous connective tissue are released by platelets, causing the adherence of material and formation of a fibrous cap and atheroma.4 The fibrous cap prevents the necrotic center of an atheroma from leaking into the vessel lumen. If the cap ruptures, however, a thrombus can form. This process subsequently allows emboli to enter distal arterioles.
Plaque accumulation tends to occur at arterial bifurcations, where blood flow velocity, lumen size, and shearing stress are decreased. A similar anatomical construction is believed to facilitate Hollenhorst plaque deposition in the retina, given that the plaques commonly are noted at retinal arterioles bifurcations.5 To better understand how retinal emboli occur, knowledge of the extracranial arterial system is essential. The left common carotid stems from the subclavian artery, whereas the right common carotid artery arises directly from the aortic arch. The common carotid branches into the internal and external carotid arteries to perfuse separate regions in the brain.
The first branch of the internal carotid artery is the ophthalmic artery, which ultimately forms the central retinal and posterior ciliary arteries.6 Occlusions secondary to emboli typically occur in the central retinal artery and its branches. Researchers have postulated that cholesterol plaques are seen in the retina due to their small size and decreased velocity while traveling through the internal carotid system.7 Considering the small diameter of the ophthalmic artery, larger emboli with increased velocities simply may bypass the ophthalmic branch.
• Diagnostic testing. The presence of a Hollenhorst plaque on funduscopy typically merits further evaluation to rule out underlying systemic vascular disease. Minimal baseline work-up for ophthalmic providers consists of a lipid panel, carotid auscultation and carotid duplex. Transesophageal echocardiography and computed tomographic angiography (CTA) also may be necessary for further evaluation, particularly if the initial work-up is negative or the findings are atypical.
A carotid duplex is a non-invasive imaging technique that uses both B-scan ultrasonagraphy and doppler ultrasonography to evaluate the common, internal and external carotid arteries. Duplex imaging rapidly alternates between the two methods, providing the technician with an accurate determination of blood flow velocity and arterial plaque formation.9 Although the procedure is heavily dependent on the technician, duplex imaging is highly accurate when compared to the gold standard of arteriography.10
Evaluation of the carotid arteries provides information about the peak systolic velocity, end diastolic velocity, diameter reduction, plaque size and morphology, spectral characteristics, and direction of vertebral flow. It is typical to note an increased peak systolic velocity with a larger degree of stenosis (as the vessel lumen decreases, blood is forced through at a higher velocity).
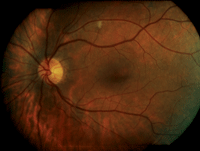
2. Color fundus photo montage of the left eye at follow up #1 on June 22, 2011. Note the isolated microinfarct located along the superior temporal arcade as well as the resolved Hollenhorst plaques.
Significant internal carotid artery stenosis is characterized by a vessel diameter reduction of 80% to 99%, a peak systolic velocity greater than 125cm/sec, an end diastolic velocity greater than 140cm/sec and extensive spectral broadening.9
CTA typically is performed to accurately determine the degree of carotid stenosis prior to surgical intervention. The procedure uses an intravenous contrast material, which facilitates clear imaging of the arterial lumen.
CTA provides thin-slice images in different planes, which can be compiled into a single, three-dimension image. Measurements for stenosis determination are made via the narrowest portion of the stenosed lumen as well as distally (where the lumen is believed to be normal).11
When performed on patients with stenosis percentages between 70% and 99%, CTA has a sensitivity of 85% and specificity of 93%.12 Such testing is especially beneficial when duplex results are suggestive of total occlusion.
The majority of retinal cholesterol emboli typically originate from the internal carotid artery. Be certain to consider transesophageal echocardiography in young patients and when an emboli of cardiac origin is suspected.13
Although the majority of cardiac emboli will be calcific or fibroid, the aortic arch also may develop atheromas.14 In contrast to Hollenhorst plaques, larger cardiac emboli often are more visually devastating because they tend to lodge in the central retinal artery.
Carotid artery evaluation with duplex ultrasonography may be ordered for ocular etiologies such as transient monocular vision loss, retinal venous occlusions, retinal artery occlusions, optic atrophy, peripheral retinal hemorrhaging, asymmetric diabetic retinopathy, venous stasis retinopathy, normal-tension glaucoma, retina emboli and ocular ischemic syndrome (see “A Review of Ocular Ischemic Syndrome,” below.).15,16
Given this extensive list of conditions, you must be aware of the positive predictive value of the findings as they relate to the likelihood of significant carotid stenosis.
Vicki Lyons-Wait, OD, and associates summarized the incidence of hemodynamically significant stenosis based solely on ocular risk factors.15 Of particular interest to this report, the prevalence of hemodynamically significant internal carotid artery stenosis––as found with duplex imaging––has been shown to range from 7% to 20% for asymptomatic retinal emboli and 20% to 25% for branch retinal artery occlusions.15-20
Ultimately, Dr. Lyons-Wait’s research team recommended a baseline duplex for asymptomatic patients over the age of 60 with comorbid systemic vascular conditions and evidence of pertinent retinal findings.15
Heath K. McCullough, MD, and associates found similar moderate positive predictive values of carotid stenosis for the ocular signs/symptoms of amaurosis fugax (18.2%), Hollenhorst plaques (20.0%) and venous stasis retinopathy (20.0%).21
| A Review of Ocular Ischemic Syndrome
|
| No discussion of the carotid system would
be complete without discussing ocular ischemic syndrome (OIS).
Widespread ischemia to the eye may result when the carotid artery is at
least 90% occluded. Patients may experience decreased vision,
periorbital eye pain, headache, amaurosis fugax and extended visual
recovery after photostress. Anterior segment findings may include
episcleral injection, corneal edema, iris neovascularization and uveitis
with increased flare in the anterior chamber. Posterior segment signs
of OIS include arteriolar narrowing, venous dilation without tortuosity,
midperipheral retinal hemorrhages, microinfarcts and optic disc/retinal
neovascularization.31,32 Although the most common symptom of
OIS is decreased vision, patients may present with mixed signs and
symptoms. Because many of these findings are noted in other ocular
conditions, clinicians should consider OIS when ocular findings are
asymmetrical.
The intraocular pressure can range from low to high, depending on the state and duration of OIS. Low unilateral eye pressure is indicative of hypoperfusion to the ipsilateral ciliary body. Significantly elevated intraocular pressure may be due to neovascular glaucoma from widespread ocular ischemia. Although neovascular glaucoma should initially be treated with topical medical therapy, surgical intervention may be necessary if the intraocular pressure is not reduced to an appropriate level.31 The treatment of OIS is targeted at fixing the underlying etiology of the carotid artery or other atherosclerotic sites. Surgical procedures include carotid endarterectomy (CEA) and carotid angioplasty and stenting (CAS). While there are no large clinical studies documenting the neovascular and visual changes after CEA or CAS, some case reports and small-scale studies have shown resolution of neovascularization within several days of CEA. Other case reports have also shown that OIS retinopathy without neovascularization can improve following CEA. It is important to note that surgical intervention has never been shown to reverse chronic neovascular glaucoma.33-35 Local
ocular treatment is targeted at retinal and iris neovascularization.
Panretinal photocoagulation (PRP) has produced mixed results when used
to treat OIS. PRP has been shown to successfully reduce ocular
neovascularization, but has a minimal effect on visual acuity due to
chronic ischemia of the retina.36-39 Intravitreal injections
of anti-vascular endothelial growth factor also have been found to
reduce retinopathy, iris neovascularization and neovascular glaucoma
secondary to OIS.40,41
One study revealed that two individuals with iris neovascularization and cystoid macular edema exhibited a drastic improvement in signs and symptoms following initial bevacizumab injection. Despite clinical improvement of the macular edema, the authors documented no improvement in best-corrected visual acuity.40 |
Treatment Options
The treatment decision for carotid stenosis largely is based on the degree of stenosis and history of symptoms. Patients with less than 50% stenosis typically are managed medically with the use of anti-platelet aggregates, while those with more than 70% stenosis may be considered surgical candidates.
• Therapeutic intervention. Each antiplatelet medication features a different mechanism of action. Some of the more common antiplatelet medications include aspirin (COX-2 inhibitor), clopidogrel (adenosine diphosphate [ADP] receptor inhibitor) and dipyridamole (ADP reuptake inhibitor). Despite varying mechanisms, the primary goal of any antiplatelet medication is to reduce the aggregation of platelets near a diseased site and the limit the formation of additional atheromas. Antiplatelet therapy has been shown to reduce the five-year stroke rate by approximately 50% in asymptomatic stenosis.22 Anticoagulants also have been evaluated as primary medical therapy; however, there has been no data supporting the superiority of such medications (e.g., warfarin) over conventional antiplatelet therapy.
Intense cholesterol lowering has long been postulated to reduce the likelihood of both fatal and non-fatal strokes. Subgroup analysis in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels trial indicated that intense lipid reduction with atorvastatin reduced the risk of both cerebrovascular and cardiovascular events in patients with carotid stenosis.23 Despite the retrospective and non-randomized nature of this review, the data suggest that patients with known carotid stenosis may benefit from statin use as well as antiplatelet therapy.
• Surgical intervention. Carotid endarterectomy is performed under general or localized anesthesia. After an incision is made along the diseased arterial portion, a tubing shunt is placed between the proximal and distal ends to permit blood flow to the brain during the procedure. The atheromatous plaque is then excised and the artery is closed.24
Carotid angioplasty and stenting is another procedure that is gaining popularity due to the surgical risks associated with endarterectomy. CAS consists of an arteriogram of the carotid artery followed by placement of a filter known as a cerebral protective device, which prevents debris and emboli from traveling to the ipsilateral cerebral hemisphere. The balloon is then inserted to dilate the artery and a stent is inserted to recanalize the blood flow.24,25
A landmark study comparing the two procedures was completed in 2010. Although CEA and CAS were both found to be safe and effective in treating carotid artery stenosis, there was a higher risk of myocardial infarction following CEA and a greater risk of stroke after CAS.26
Both therapeutic and surgical treatments have advantages and disadvantages. Studies have examined the most appropriate treatment, given the degree of stenosis, symptomatology and medical status. The North American Symptomatic Carotid Endarterectomy Trial showed that surgical intervention was markedly more effective than medical therapy in patients with 70% to 99% stenosis.27 However, in those with just 50% to 69% stenosis, the benefits of surgery dropped considerably. Further, there was no documented benefit of CEA in patients with less 50% stenosis. Instead, the researchers recommended medical therapy for these individuals.27 Similar results were obtained in symptomatic patients in the European Carotid Surgical Trial.28
The Asymptomatic Carotid Atherosclerosis Study and Asymptomatic Carotid Surgery Trial showed a risk reduction of 53% and 46%, respectively, when CEA was performed on patients with minimum stenosis of 60%.29,30 Guidelines from these studies only recommend CEA when the risk of perioperative stroke, myocardial infarction or mortality was low. The authors determined that patients with less than 60% stenosis are best managed on medical therapy alone, until they become symptomatic or the degree of stenosis increases to more appropriate surgical levels.29,30
This case illustrates the significance of retinal cholesterol emboli and their relation to carotid artery disease. Patient history, retinal findings and imaging studies help determine whether surgical intervention may be beneficial to a patient. Clinicians should consider a duplex ultrasound for individuals who present with ocular signs, neurologic symptoms or comorbid vascular conditions.
Although CEA typically is not indicated for asymptomatic patients who exhibit less than 60% stenosis, medical therapy should be considered to prevent further plaque accumulation and increased stenosis. Both CEA and CAS have associated perioperative risks, but are relatively safe options for individuals who require surgical intervention for hemodynamically significant internal carotid stenosis.
Dr. Zimbalist practices at the Harry S. Truman Memorial Veterans’ Hospital in Columbia, Mo.
1. Hollenhorst RW. Ocular manifestations of insufficiency or thrombosis of the internal carotid artery. Trans Am Ophthalmol Soc. 1958;56:474-506.
2. Sowka JW, Gurwood AS, Kabat AG. Review of Optometry: The Handbook of Ocular Disease Management. Hollenhorst Plaque. Available at:
http://cms.revoptom.com/handbook/sect5l.htm. Accessed March 12, 2013.
3. Yanoff M, Duker JS. Retinal Arterial Obstruction. Ophthalmology, 2nd ed. St. Louis: Mosby; 2004:858.
4. Marks ES, Thomann KH, Adamczyk DT. Arteriosclerosis. Primary Eyecare in Systemic Disease, 1st ed. Norwalk, CT: McGraw-Hill Professional; 1995:31-9.
5. Suri JS, Kathuria C, Molinari F. Introduction to the Pathology of Carotid Atherosclerosis: Histological Classification and Imaging Correlation. Atherosclerosis Disease Management. New York: Springer; 2011:6.
6. Netter FH. Head and Neck. Atlas of Human Anatomy, 3rd ed. Teterboro, NJ: Icon Learning System; 2004:26-133.
7. Mead GE. Comparison of risk factors in patients with transient and prolonged eye and brain ischemic syndromes. Stroke. 2002 Oct;33(10):2383-90.
8. Saric M, Kronzon I. Cholesterol embolization syndrome. Curr Opin Cardiol. 2011 Nov;26(6):472-9.
9. Gillard JH, Graves MJ, Hatsukami TS, Yuan C. Conventional Carotid Doppler Ultrasound. Carotid Disease: The Role of Imaging in Diagnosis and Management. New York: Cambridge University Press; 2007:105-11.
10. Shakhnovich I, Kiser D, Satiani B. Importance of validation of accuracy of duplex ultrasonography in identifying moderate and severe carotid artery stenosis. Vasc Endovascular Surg. 2010 Aug;44(6):483-8.
11. Nadalo LA. Carotid artery stenosis imaging. Medscape. Available:
http://emedicine.medscape.com/article/417524-overview#a20. Accessed March 12, 2013.
12. Koelemay MJ, Nederkoorn PJ, Reitsma JB, Majoie CB. Systematic revoew of computed tomographic angiography for assessment of carotid artery disease. Stroke. 2004 Oct;35(10):2306-12.
13. Marks ES, Thomann KH, Adamczyk DT. Cardiogenic Emboli and Valvular Heart Disease. Primary Eyecare in Systemic Disease, 1st ed. Norwalk, CT: McGraw-Hill Professional; 1995:40-51.
14. Romano JG, Babikian VL, Wijman CA, Hedges TR 3rd. Retinal ischemia in aortic arch atheromatous disease. J Neuroophthalmol. 1998 Dec;18(4):237-41.
15. Lyons-Wait VA, Anderson SF, Townsend JC, De Land PD. Ocular and systemic findings and their correlation with hemodynamically significant carotid artery stenosis: a retrospective study. Optom Vis Sci. 2002 Jun;79(6):353-62.
16. Bull DA, Fante RG, Hunter GC, et al. Correlation of ophthalmic findings with carotid artery stenosis. J Cardiovasc Surg (Torino). 1992 Jul-Aug;33(4):401-6.
17. Bruno A, Russell PW, Jones WL, et al. Concomitants of asymptomatic retinal cholesterol emboli. Stroke. 1992 Jun;23(6):900-2.
18. O’Donnell BA, Mitchell P. The clinical features and associations of retinal emboli. Aust N Z J Ophthalmol. 1992 Feb;20(1):11-7.
19. Chawluk JB, Kushner MJ, Bank WJ, et al. Atherosclerotic carotid artery disease in patients with retinal ischemic syndromes. Neurology. 1988 Jun;38(6):858-63.
20. Dunlap AB, Kosmorsky GS, Kashyap VS. The fate of patients with retinal artery occlusion and Hollenhorst plaque. J Vasc Surg. 2007 Dec;46(6):1125-9.
21. McCullough HK, Reinert CG, Hynan LS, et al. Ocular findings as predictors of carotid artery occlusive disease: Is carotid imaging justified? J Vasc Surg. 2004 Aug;40(2):279-86.
22. Mohan KM, Wolfe CD, Rudd AG, Heuschmann PU, et al. Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke. 2011 May;42(5):1489-94.
23. Amarenco P, Bogousslavsky J, Callahan A 3rd, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006 Aug 10;355(6):549-59.
24. Doig D, Brown MM. Carotid stenting versus endarterectomy. Annu Rev Med. 2012;63:259-76.
25. Faisal A. Carotid artery stenting. Medscape. Available at:
http://emedicine.medscape.com/article/1839544-overview#. Accessed March 12, 2013.
26. Brott TG, Hobson RW 2nd, Howard G, et al. Stenting versus Endarterectomy for Treatment of Carotid-Artery Stenosis. N Engl J Med. 2010 Jul 1;363(1):11-23
27. Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998 Nov 12;339(20):1415-25.
28. European Carotid Surgery Trialists’ Collaborative Group. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998;351(9113):1379-87.
29. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995 May 10;273(18):1421-8.
30. Halliday A, Mansfield A, Marro J, et al. MRC Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomized controlled trial. Lancet. 2004 May 8;363(9420):1491-502
31. Kaiser PK, Friedman NJ. Retina and Choroid. The Massachusetts Eye and Ear Infirmary Illustrated Manual of Ophthalmology, 2nd ed. Philadelphia: Saunders; 1998:307-8.
32. Chen CS, Miller NR. Ocular ischemic syndrome: Review of clinical presentations, etiology, investigation, and management. Compr Ophthalmol Update. 2007 Jan-Feb;8(1):17-28.
33. Neupert JR, Brubaker RF, Kearns TP, Sundt TM. Rapid resolution of venous stasis retinopathy after carotid endarterectomy. Am J Ophthalmol. 1976 May;81(5):600-2.
34. Ino-ue M, Azumi A, Kaijura-Tsukahara Y, Yamamoto M. Ocular ischemic syndrome in diabetic patients. Jpn J Ophthalmol. 1999 Jan-Feb;43(1):31-5.
35. Geroulakos Gl. Effect of carotid endarterectomy on the ocular circulation and on ocular symptoms unrelated to emboli. Eur J Vasc Endovasc Surg. 1996 Apr;11(3):359-63.
36. Carter JE. Panretinal photocoagulation for progressive ocular neovascularization secondary to occlusion of the common carotid artery. Ann Ophthalmol. 1984 Jun;16(6):572-6.
37. Eggleston TF, Bohling CA, Eggleston HC, et al. Photocoagulation for ocular ischemia associated with carotid artery occlusion. Ann Ophthalmol 1980;12:84.
38. Johnston ME, Gonder JR, Canny CL. Successful treatment of the ocular ischemic syndrome with panretinal photocoagulation and cerebrovascular surgery. Can J Ophthalmol. 1988 Apr;23(3):114-9.
39. Chen KJ, Chen SN, Kao LY, et al. Ocular ischemic syndrome. Chang Gung Med J. 2001 Aug;24(8):483-91.
40. Amselem L, Montero J, Diaz-Llopis M, et al. Intravitreal bevacizumab (Avastin) injection in ocular ischemic syndrome. Am J Ophthalmol. 2007 Jul;144(1):122-4.
41. Wakabayashi T, Oshima Y, Sakaguchi H, et al. Intravitreal bevacizumab to treat iris neovascularization and neovascular glaucoma secondary to ischemic retinal diseases in 41 consecutive cases. Ophthalmology. 2008 Sep;115(9):1571-80.

