Annual Dry Eye ReportCheck out the other feature articles in this month's report: |
In recent years, diagnosis, management and treatment of dry eye disease (DED) have expanded greatly. Our understanding of what makes an eye “dry” has grown from simply stating that a patient has dry eye to understanding the many factors that can contribute to its etiology.
While emerging treatments largely focus on evaporative dry eye (EDE), aqueous deficiency can exist independently or even concurrently in patients who have meibomian gland dysfunction (MGD). Make sure you don’t overlook this uncommon but impactful condition while evaluating a patient for dry eye. The following article reviews aqueous-deficient dry eye (ADDE) and corresponding diagnostic and management options.
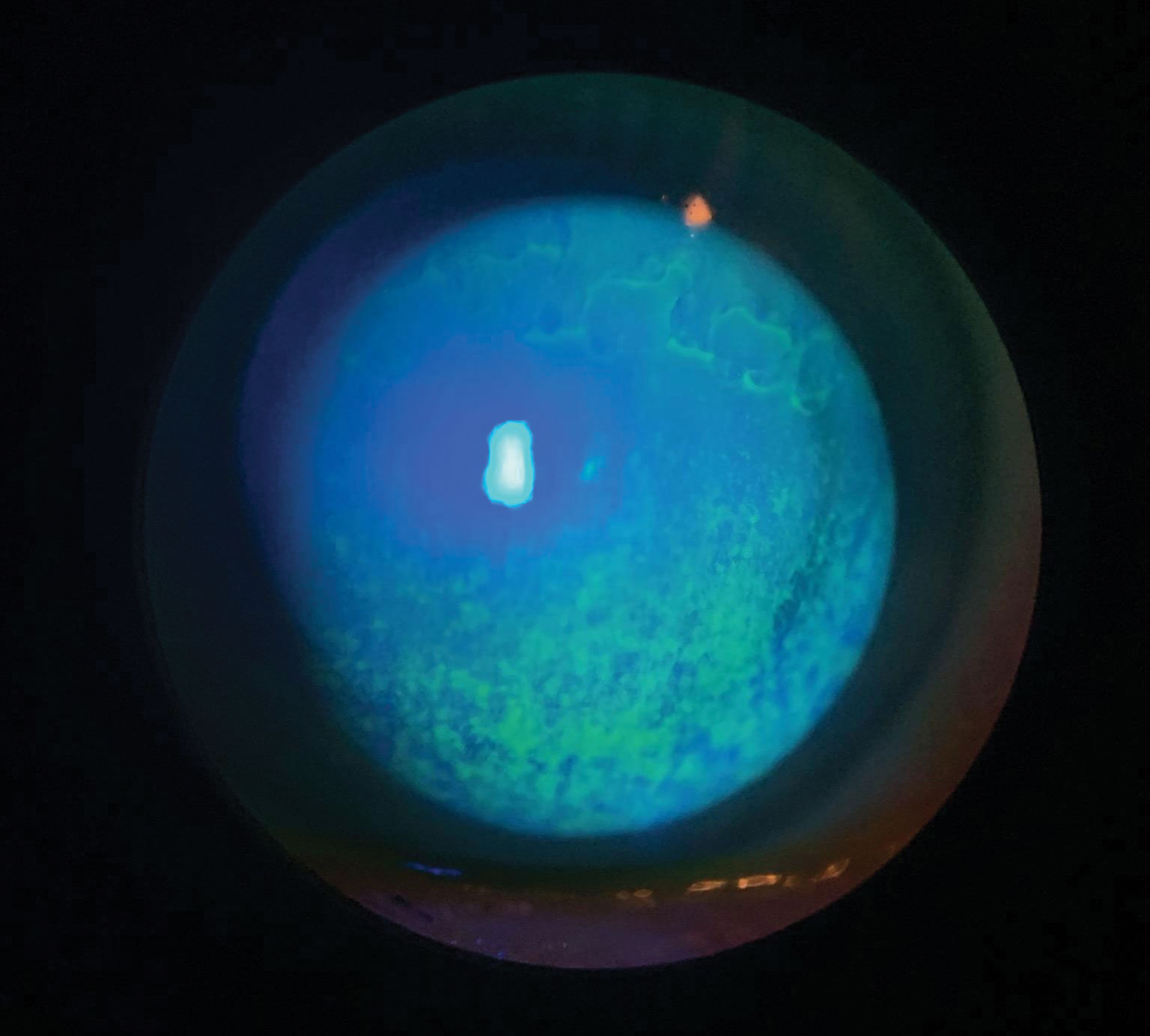 |
| Patients with punctate corneal staining—a common finding in ADDE—can be asymptomatic or have severe pain and decreased vision. Click image to enlarge. |
Dry Eye Subsets
The Tear Film and Ocular Surface Society (TFOS) defines DED as “a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage and neurosensory abnormalities play etiological roles.”1
The two predominant etiologies, ADDE and EDE, can present as single and separate processes or may overlap with each other. The signs and symptoms of both affect the entire ocular surface, including the tear film, cornea, conjunctiva, eyelids and lacrimal and meibomian glands.2
EDE is caused by MGD, and ADDE results from reduced aqueous secretion. EDE, and cases of DED that involve EDE and ADDE, accounts for over 80% of all dry eye cases. Only about 10% of DED is classified as strictly ADDE.3
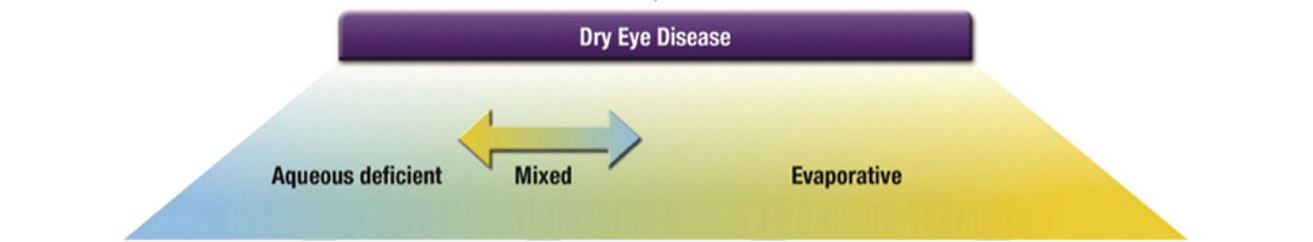 |
| DED is on a continuum of pathophysiology and is not a dichotomous disease. Image: TFOS DEWS II. Click image to enlarge. |
Aqueous Deficiency
ADDE can be divided into Sjögren’s syndrome (SS) and non-SS.4
Sjögren’s. This chronic autoimmune disorder is characterized by immune cell infiltration of the exocrine glands and systemic complications due to autoantibody production.4 SS predominantly affects women and may be due to an abnormal immune response to environmental or viral triggers in susceptible patients.4 It mainly targets the lacrimal and salivary glands and can cause gland destruction and signs and symptoms of dry eye and mouth.4
Serological testing for SS is geared toward detecting the presence of autoantibodies, specifically anti-SSA and anti-SSB. Earlier stages of SS can be detected through biomarkers for anti-salivary protein-1, anti-carbonic anhydrase and parotid-specific protein.5,6 You can order these tests, along with other rheumatologic markers, such as anti-nuclear antibodies, rheumatoid factor, erythrocyte sedimentation rate and c-reactive protein.5 The gold standard for SS diagnosis has long been salivary gland biopsy, but ultrasound imaging is emerging as a potential technique for earlier detection.5-7
Non-Sjögren’s. ADDE without systemic autoimmune features of SS could involve congenital or acquired forms of DED.7 Causes of non-SS dry eye include lacrimal gland ablation, congenital alacrima, triple A syndrome and ocular surface aging. Systemic conditions, such as sarcoidosis and lymphoma, and viral infections, such as hepatitis C and HIV/AIDS, are also potential causes.7 Direct damage to the lacrimal gland due to radiation therapy of the head/neck, chemical injury or cicatricial changes resulting from graft-vs.-host disease, Stevens-Johnson syndrome, trachoma or ocular pemphigoid may lead to ADDE.7 Another contributing factor includes decreased corneal sensation, which can be triggered pharmacologically, after refractive surgery or secondary to disease.7
Signs and symptoms are similar in non-SS and SS.4 However, the onset of dry eye is later in non-SS patients. Non-SS patients also tend to be older and have less severe disease with a smaller risk of blindness.4
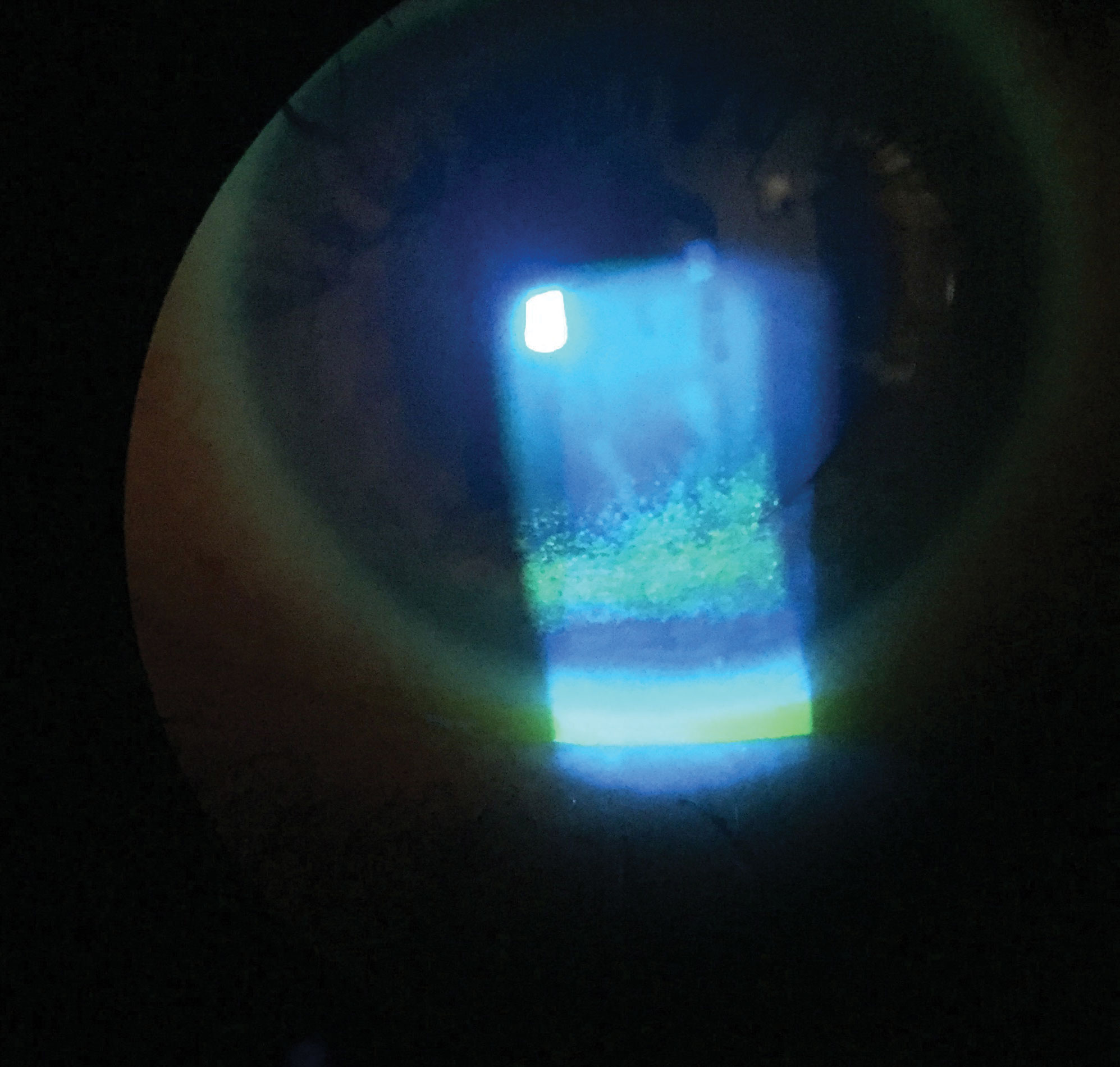 |
| This patient has extensive punctate epithelial keratitis and decreased TBUT. Click image to enlarge. |
General Testing
DED testing is the same for all cases. There are three key diagnostic tests the DEWS II recommends in symptomatic patients who have screened positive on patient questionnaires, such as the Dry Eye Questionnaire 5 (score >6) or the Ocular Surface Disease Index (score >13) (OSDI).
Tear breakup time. Noninvasive tear breakup time (TBUT) is always recommended over traditional fluorescein TBUT to minimize the dye’s impact on tear film stability. However, if fluorescein is used, the test strip should be dry and applied to the outer canthus to decrease any irritation of the ocular surface, and measurements should be taken one to three minutes after instillation.8 Noninvasive TBUT is measurable with a corneal topographer.8 A positive test result is a TBUT of less than 10 seconds after the patient blinks.
Tear hyperosmolarity. Out of all the available clinical tests, this marker has the highest correlation to DED severity. The DEWS II states that tear osmolarity is the single best metric to diagnose and classify DED.8 A positive result is any reading greater than 308mOsm/L or a difference of more than eight between eyes. Although the pathophysiology of patients with EDE or ADDE differs, the tear osmolarity and distribution do not.9
Ocular surface staining. Conjunctival and lid margin damage is best viewed with lissamine green staining, and corneal damage is more visible with fluorescein dye.8 Rose bengal concentrates on corneal and conjunctival cells that lack mucin and can identify damaged epithelial cells.9 Staining in either eye confirms a positive result that commonly indicates late-stage DED. Ocular surface staining is defined as more than five corneal or nine conjunctival spots or lid margin staining (lid wiper epitheliopathy) greater than 2mm in length.
While DEWS II suggestions for initial DED testing do not include tear volume, this parameter plays an important role in assessing ocular surface health and homeostasis, particularly in patients with aqueous deficiency.8 Decreased tear volume may be a key pathogenic mechanism and diagnostic sign of ADDE patients who suffer independently from EDE.8
Tear volume can be indirectly evaluated noninvasively via tear meniscus assessment, which can help with DED subclassification.8 The meniscus is best viewed in the center of the lower eyelid shortly after a blink. The simplest way to grade it is to conduct meniscometry at the slit lamp by judging the meniscus height in comparison with the slit lamp beam height, but this method has poor inter-visit repeatability.8 Digital measurement techniques are emerging and exhibit better reproducibility but are not mainstay at this time.8
Perform Schirmer’s testing by inserting the paper strip at the temporal one-third of the lower lid margin and measuring the length of wetting after five minutes either with or without anesthesia. A Schirmer’s test result >10mm is normal, between 5mm and 10mm is borderline and <5mm is indicative of aqueous deficiency.8 Conducting the test without an anesthetic is best to confirm severe ADDE, but its variability and invasiveness precludes its use as a routine diagnostic test of tear volume, especially in cases with concurrent EDE where tear quality rather than quantity is predominantly affected.8 Under these circumstances, testing can stimulate a reflex tearing response on insertion of the testing strip and mask results.8
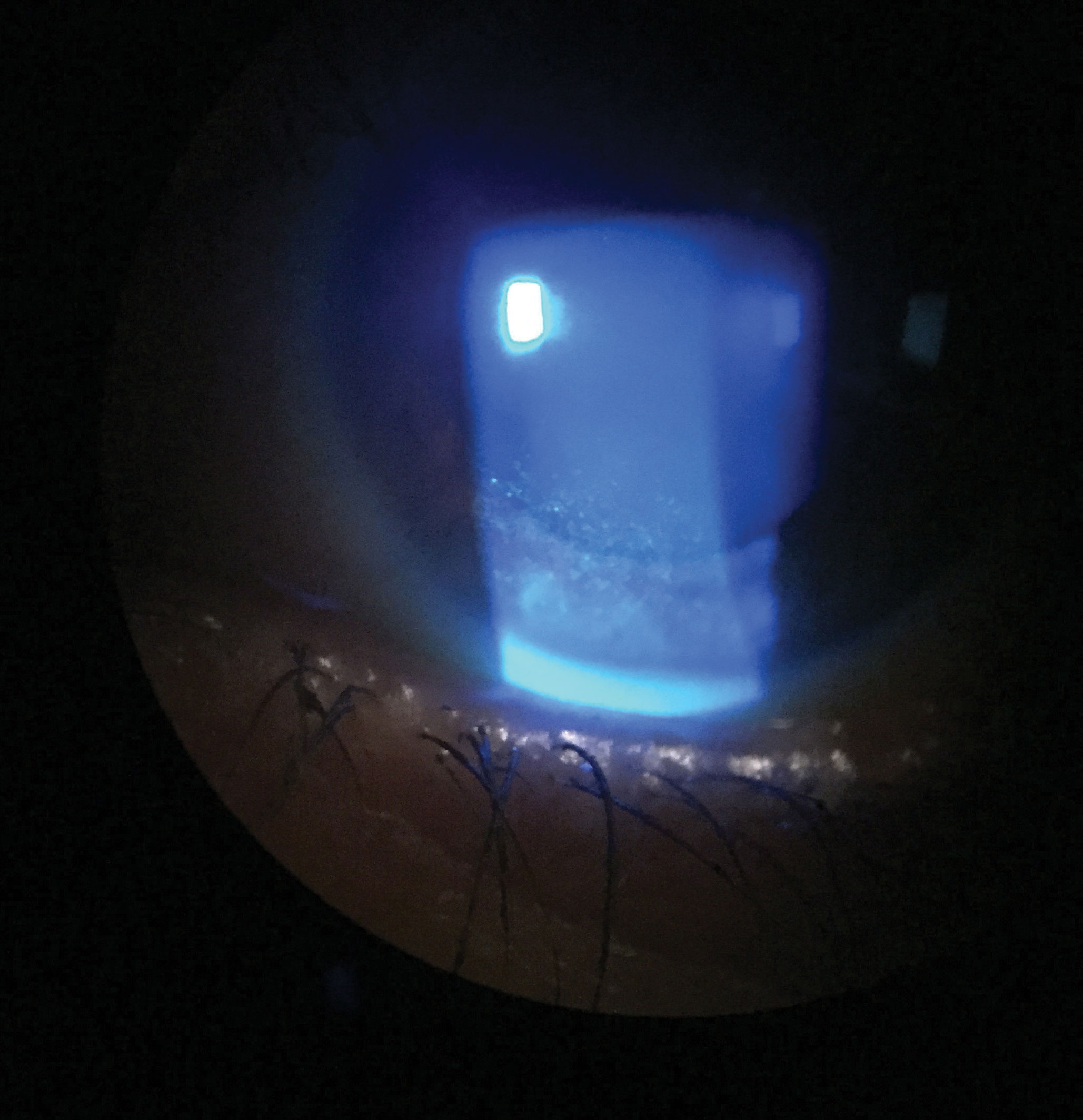 |
| The patient improved after four months of treatment with topical lubrications, a tapered course of topical steroids and lifitegrast twice daily. Click image to enlarge. |
Treatment Options
Historically, ADDE was treated by prescribing tear replacement products or by conserving tears via punctal plugs.10 While these methods still play a key role in treating ADDE, recent treatments focus on better stimulating tear production.10
Managing dry eye often requires several simultaneous methods of care. Following a patient’s signs and symptoms to gauge the efficacy of their current treatment regimen is key when deciding whether additional therapy is necessary.
Artificial tears. These over-the-counter products do not address the pathophysiology of DED but can offer temporary relief.10 Products vary in osmolarity, viscosity and pH. Higher viscosity agents, such as carboxymethyl cellulose, hyaluronic acid, HP-guar, polyvinyl alcohol and propylene glycol, are typically reserved for overnight use. Viscosity enhancers are beneficial to the ocular surface, as they increase tear film thickness, shield against desiccation, promote tear retention at the ocular surface, protect the ocular surface, maintain physiological corneal thickness, improve goblet cell density and relieve dry eye symptoms. Osmoprotectants, such as trehalose—which can be found in TheraTears Extra (Akorn) and Refresh Optive Mega-3 (Alcon)—can protect corneal cells from desiccation and high osmolarity by fortifying cell membranes.11,12
Topical pharmaceutical agents. Cyclosporine A is an immunomodulatory drug with anti-inflammatory properties that inhibits the IL-2 activation of lymphocytes.10 Topical cyclosporine was approved by the FDA for moderate to severe DED and can improve inflammation, tear osmolarity, conjunctival goblet cell density and tear production.10 It is available in three preservative-free forms: Restasis (cyclosporine A 0.05%, Allergan), Cequa (cyclosporine A 0.9%, Sun Pharmaceuticals) and Klarity-C (cyclosporine A 0.1%, Imprimis Rx).
Xiidra (lifitegrast, Novartis) is a small-molecule integrin antagonist that binds to cell surface proteins found on leukocytes and blocks the integrin lymphocyte function-associated antigen-1 and cognate ligand intercellular adhesion molecule-1 interactions.13 In vitro studies have shown that Xiidra may inhibit the recruitment of previously activated T-cells, the activation of newly recruited T-cells and the release of pro-inflammatory cytokines, interrupting the perpetual cycle of inflammation that promotes DED.13
Often, low-dose topical steroids, such as Lotemax SM (loteprednol etabonate ophthalmic gel 0.38% and 0.5%, Bausch + Lomb), are used as a pretreatment or concomitantly with cyclosporine or lifitegrast earlier on and tapered off after a few weeks. Preservative-free steroids, such as dexamethasone 0.01%—which is currently unavailable in the US—can improve symptoms of chronic ocular surface irritation that were previously unresponsive to preserved topical steroids, such as loteprednol 0.2%, fluorometholone 0.1% and prednisolone 1%.10
Biological tear substitutes. In more advanced cases of DED where topical pharmaceutical agents do not provide adequate relief of severe ocular surface disease, as is the case with many ADDE patients, autologous serum is an effective option. It is derived from the biomarkers in a patient’s own blood and contains many biochemical characteristics similar to that of human tears, such as pH level, nutrients, vitamins, albumin, fibronectin and epithelial and nerve growth factors.10 Autologous serum, and other blood-derived tear substitutes, can promote corneal epithelial wound healing, inhibit the release of inflammatory cytokines, increase the number of goblet cells and encourage mucin expression.10
Specialized compounding pharmacies formulate autologous serum, the concentration of which depends on symptom severity, after the patient’s blood is drawn and screened for HIV, hepatitis and other conditions.10
Typically kept frozen for up to nine months at -20°C, a defrosted vial is good for about 24 hours after it thaws, as it is non-preserved.10 A study found that treatment with autologous serum can improve patient symptoms in as soon as 10 days in 60% of patients and two months in 79%.10 While patient symptoms, TBUT and corneal staining improved, Schirmer’s scores remained the same, and ocular surface disease recurred after discontinuation of treatment.10
Punctal occlusion. ADDE patients benefit from aqueous retention on the ocular surface, and, as such, temporary or permanent punctal occlusion of one or both puncta at the level of the punctal opening or deeper can be an appropriate treatment.10 Commonly, punctal occlusion is performed via punctal plugs. Absorbable, collagen-based plugs with absorption rates that vary from one week to six months are the most commonly used. Non-absorbable, or “permanent,” plugs are typically silicone-based. For advanced cases, permanent surgical closure of the puncta via total or partial thermal cauterization, occlusion with a conjunctival flap, punctal suturing or total destruction of the canaliculus can be performed.
Punctal occlusion is most successful when combined with other DED treatments.10 Occluding the puncta in the presence of ocular surface inflammation is controversial, as there is a concern that occlusion of tear outflow can prolong the presence of pro-inflammatory cytokines on the ocular surface. As such, treating surface inflammation is recommended prior to occlusion.
Moisture chamber glasses. These are specially designed to slow tear evaporation by providing a humid environment and minimizing airflow over the ocular surface.10 They are a potential adjunct to prescribed treatment for increased symptom relief.10
Topical aqueous secretagogues. Diquas (diquafosol tetrasodium 3% ophthalmic solution, Santen) is a purinergic P2Y2 receptor agonist that stimulates water and mucin secretion from conjunctival epithelial and goblet cells and improves tear film stability.10 It is only approved overseas as of now. Studies have shown topical diquafosol significantly improves corneal and conjunctival staining, TBUT and Schirmer’s scores.10 Topical lacritin, a glycoprotein that stimulates pro-secretory activity in the lacrimal gland, has therapeutic potential in treating ADDE in SS patients with reduced lacritin levels.10
Oral secretagogues. Oral pilocarpine and cevimeline, both cholinergic agonists, are commercially available to treat SS.10 While Sjögren’s patients who participated in a recent study noticed more improvement in oral vs. ocular dryness with the secretagogues, the drugs did improve ocular surface staining, goblet cell density and TBUT.10 Cevimeline had a better side effect profile than pilocarpine.10 However, there was no observed improvement in tear production with either medication.10
Neurostimulation. Intranasal tear neurostimulation induces normal tear production by stimulating the nasolacrimal reflex.10 The nasolacrimal reflex up-regulates tear production following chemical or mechanical stimulation of the nasal mucosa.10 The TrueTear Intranasal Tear Neurostimulator (Allergan) temporarily increases tear production during neurostimulation in adults. This device consists of a handheld stimulator unit equipped with a disposable two-pronged hydrogel tip and an external charger.10 It allows self-delivery of minute electrical currents with variable waveform to the anterior ethmoidal nerve, stimulating immediate natural tear production.10 Preliminary testing shows increased Schirmer’s scores and decreased patient symptoms.10
Diagnostic Criteria of SSThese American-European Consensus Classification Criteria for SS were published in 2002:14
In 2012, the American College of Rheumatology proposed simplified diagnostic criteria:15
Any patient suspected of having SS should be referred to rheumatology for further systemic evaluation and treatment. |
Advanced Strategies
For more advanced or temperamental cases, there are a few options available at your disposal.
Therapeutic contact lenses. Bandage lenses can improve ocular comfort, maintain corneal integrity and prolong ocular surface moisturization by reducing the effects of an adverse environment.10 The availability of silicone hydrogel lenses, along with their high oxygen permeability, has helped promote their use as a therapeutic device in the management of many ocular surface diseases, including DED.10 Soft lenses are typically used on an extended wear basis. A recent study shows that, in Sjögren’s patients, the use of silicone hydrogel lenses used as bandage contact lenses provided significant improvement in visual acuity for up to six weeks after discontinuing wear, OSDI scores, TBUT and corneal staining.10
Gas permeable scleral lenses are used in cases of moderate to severe DED and aim to provide a repository of tears and effective protection to the ocular surface.10 A new solution called Nutrifill (Contamac) contains essential ions found in tears. Unfortunately, once the neuropathic pain has centralized, a bandage lens may be insufficient for reducing symptoms.10
Amniotic membrane grafts. These can successfully treat severe DED with persistent epithelial defects or corneal ulceration and scarring. They consist of cryopreserved human amniotic membranes, which contain a wide variety of neuropeptides and neurotransmitters.10 Grafts are inserted similarly to a scleral lens and typically dissolve in about one week. One study shows symptom improvement for four months in dry eye patients treated with a Prokera Slim (Bio-Tissue) for five days.10
Surgical approaches. In the most severe cases of DED, surgical intervention may be necessary. Options include tarsorrhaphy, mechanical dacryoreservoirs, gland transplantation and lacrimal gland regeneration.
Tarsorrhaphy is a temporary or permanent surgical procedure that partially or totally closes the eyelids to decrease ocular surface exposure and tear evaporation in cases where all other treatments have failed.10
In patients with ADDE and a Schirmer’s score less than 2mm who have difficulty maintaining frequent topical lubrication or who have not found relief from less invasive measures, mechanical devices called dacryoreservoirs may be an option.10 They deliver lubricating or pharmaceutical agents from a reservoir through a silicone catheter that continuously lubricate the ocular surface.10 While they improve TBUT, corneal staining and conjunctival hyperemia, there is a risk of infection in the reservoir and device that may require its removal.10
Transplantation of functioning exocrine tissue from one of the three major salivary glands, typically the submandibular gland, offers lubrication in cases of severe ADDE.10
Lacrimal gland regeneration is an emerging treatment option that is exploring several strategies for stem cell expansion and differentiation into lacrimal tissue for organogenesis and engraftment techniques. This method is in its beginning phases.7
As our understanding of dry eye continues to expand, so do diagnostic and treatment options that allow us to better serve these patients. While so much of the focus remains on EDE, make sure you have a sufficient understanding of ADDE so you’re better able to manage these cases in the chance they do present and work side-by-side with rheumatology for the best results.
Dr. Tolud completed a residency in ocular disease and practices at Vantage Eye Care, South Jersey Eye Physicians Division, in Columbus, NJ. She also serves as president of the New Jersey chapter of the American Academy of Optometry.
| 1. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276-83. 2. Craig JP, Nelson JD, Azar DT, et al. TFOS DEWS II report executive summary. Ocul Surf. 2017;15(4):802-12. 3. Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. 2015;112(5):71-81. 4. Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438-510. 5. Hauswirth S. Diagnosing and treating Sjögren’s syndrome. Optometry Times. August 14, 2016. [Epub ahead of print]. 6. Abd-Allah NM, Hassan AA, Omar G, et al. Evaluation of patients with dry eye for the presence of primary or secondary Sjögren’s syndrome. Clin Ophthalmol. 2019:13:1787-97. 7. Liu CY, Hirayama M, Ali M, et al. Strategies for regenerating the lacrimal gland. Curr Ophthalmol Rep. 2017;5(3):193-8. 8. Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15(3):539-74. 9. Beckman KA, Luchs J, Milner MS. Making the diagnosis of Sjögren’s syndrome in patients with dry eye. Clin Ophthalmol. 2016;10:43-53. 10. Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575-628. 11. McDonald MB, Fumuso PW. Trehalose: a novel treatment for dry eye. Healio. www.healio.com/ophthalmology/cornea-external-disease/news/print/ocular-surgery-news/%7B6d1f0d15-4321-487b-8aa8-56648bdec2d2%7D/trehalose-a-novel-treatment-for-dry-eye?page=3. July 10, 2018. Accessed April 20, 2020. 12. Horton M, Horton M, Reinhard E. Master the maze of artificial tears. Rev Optom. 2018;155 (11):48-56. 13. Karpecki P. Say hii to Xiidra. Rev Optom. August 15, 2016. [Epub ahead of print]. 14. Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61(6):554-8. 15. Shiboski SC, Shiboski CH, Criswell L, et al. American College of Rheumatology classification criteria for Sjögren’s syndrome: a data-driven, expert consensus approach in the Sjoögren’s International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken). 2012;64(4):475-87. |

