Modern cataract surgery is perceived as refractive surgery, and expectations are high. These days, patients undergoing cataract surgery live more active lifestyles, spending large amounts of time both outdoors and in front of computers or smartphones. For many, good uncorrected distance vision is not enough anymore, and complete spectacle independence is the goal.
This demand is, in part, fueled by the success stories patients hear from their friends or family members scrapping glasses and having “perfect vision” at all distances. While we don’t always see these top-notch outcomes, we are fortunate enough to live in an age when many of those demands can be met.
As optometrists take a larger role in postoperative care of cataract patients, it is imperative to stay up-to-date on intraocular lens (IOL) advancements, counseling techniques, pharmaceutical management of the post-op course and more. When careful patient selection and counseling are combined with good surgical skill and knowledgeable postoperative management, most patients should be able to achieve those boast-worthy outcomes that continue driving patients to keep their eyes in top shape.
 |
| PanOptix IOL (Alcon), the newest diffractive trifocal IOL, provides good unaided visual acuity at distance, intermediate and near. Click image to enlarge. |
Step One: Understand Your Options
It stands to reason that the doctor must have a comprehensive understanding of the technologies and techniques associated with a procedure before they can educate or even evaluate their patient for it. So, the first step to managing cataract patients is to bone up on what’s available.
IOL technology is ever-evolving, with new products entering the market on a regular basis. Since 1997, multifocal IOLs have provided similarly good distance vision as monofocals, but they surpass their predecessor when it comes to intermediate and near vision. However, the multifocal lens design can result in a higher incidence of unwanted visual phenomena such as contrast sensitivity loss, glare and halos.1-4
Many patients have benefited greatly from multifocals, but some are extremely dissatisfied with either these unwanted visual phenomena, quality of vision, or higher-than-expected dependency on corrective lenses.5
All multifocal IOLs work by separating light into different foci, causing a dispersion of light energy. Different brands use different technologies to achieve this.6 Today’s multifocals function according to one of three different optic principles: refractive, diffractive or extended-depth-of focus.7
- Refractive multifocals use concentric zones of increasing dioptric power on the anterior lens surface with highest power in the center of the lens. These are highly dependent on the patient’s pupil size, as the changes in pupil diameter affect the number of zones in use. As pupils decrease in size with the near reflex, the effective power of the lens is increased.7-8 Early examples of this design include the Array and ReZoom (both originally from AMO, now Johnson & Johnson Vision).
- Diffractive multifocals possess concentric diffractive surfaces on the posterior portion, inducing wavefront interference. This helps reduce glare and higher-order aberrations.8 This design does not completely eliminate stray light.9 About 41% of incident light is allocated to distance focus, 41% to near and 18% is directed to higher-order diffraction, rendering it unusable.10
Both refractive and diffractive designs cause an in-focus image to be overlaid by at least one out-of-focus image. This affects contrast and exacerbates haloes and glare.10
Examples of diffractive bifocal IOLs include Restor (Alcon) and Tecnis multifocal IOL (Johnson & Johnson Vision).
The newest trifocal, PanOptix (Alcon), also employs a diffractive design, but improves on it to provide better unaided intermediate vision.11-13 PanOptix uses 88% of light energy (instead of 82% with older diffractive bifocals) and reduced dependence on pupil size.14
- Extended depth-of-focus (EDOF) IOLs, such as the Tecnis Symfony (Johnson & Johnson Vision), combine a unique diffractive pattern with technology that corrects corneal chromatic aberration, resulting in an elongated depth of focus and enhanced contrast sensitivity.7,15 An extended continuous focal point is different from two or three peaks of best visual acuity with bifocal or trifocal IOLs and helps reduce overlap of near and far images.8 This improves visual quality and decreases glare and unwanted visual phenomena.8,15-17
EDOF lenses have similar photopic contrast sensitivity to monofocals, much improved from the diffractive-refractive multifocal lens design.15 Near visual acuity with EDOF lenses may be worse than with diffractive IOLs, but intermediate visual acuity is equal or superior.16-18 Targeting the non-dominant eye for -0.50D myopia may help improve near vision without significantly sacrificing distance.
Symfony IOL provides similar uncorrected distance and intermediate vision as the PanOptix IOL with the same low rate of side effects, but PanOptix offers better uncorrected near vision.18 There may be less halos and glare with PanOptix than Symfony, but the Symfony IOL may still be better at preserving contrast sensitivity.14,19
 |
| The Symfony IOL is the first extended depth of focus IOL on the market. Its design corrects chromatic aberration and enhances contrast sensitivity. Click image to enlarge. |
Step Two: Set the Stage
As innovative as these new IOL options are, patients should be properly educated that they aren’t perfect—and may not be a perfect match for them. For starters, they’ll want to know a large out-of-pocket cost is usually associated with premium IOLs. Be aware that this large price tag may actually elevate their expectations, too. The most important component of post-op success with a premium IOL is preoperative counseling. After all, it is much easier to prevent undesirable outcomes than to fix them after the fact. Implantation of multifocals without discernment or discretion may yield many disgruntled patients. Patients who elect a presbyopia-correcting IOL must be motivated to be spectacle independent. Those who place high value on the improvement in range may be more likely to accept potential small loss in contrast sensitivity or temporary dysphotopsia.
The importance of a thorough discussion with a patient prior to surgery cannot be stressed enough. The conversation should include a careful evaluation of patient’s needs, lifestyle and personality. This is where the patient’s primary optometrist has an edge over the surgery clinic. The optometrist may already have a relationship and know the patient well, having a better sense of the patient’s expectations. Asking about lifestyle, work, hobbies will give information about the types of visual tasks patient performs. Personality assessment may help estimate the ability to neuroadapt.
Patients with unrealistic expectations or an overly critical personality are less likely to fare well with premium IOLs.
Patients’ refractive error and current visual acuity should be considered. Hyperopes who have significant cataracts will gain the most from presbyopia-correcting IOLs, with uncorrected vision improvement at all distances. Mild myopes who rely on their near vision for specific tasks may have something to lose and could be dissatisfied with the result.
About 35% to 40% of eyes undergoing cataract surgery have astigmatism equal to or more than 1.0D and about 20% have astigmatism greater than 1.5D.20-22 It is of great importance that most of the astigmatism is corrected. Anything more than 0.75D of postoperative cylinder will cause a significant decline in visual quality.23 Regular corneal astigmatism with good repeatability across measurement devices is ideal and can be reliably corrected with either the implantation of a toric intraocular lens or limbal relaxing incisions.
Eyes with corneal conditions—such as keratoconus, anterior basement membrane dystrophy or corneal scars—are not good candidates for premium IOLs due to the risk of higher-order aberrations and irregular astigmatism.
Ocular surface disease (OSD) should be addressed both preoperatively and postoperatively. Even if the patient was previously asymptomatic, OSD findings may bring about symptoms after surgery. Patients with variable corneal measurements or visible dry eyes may benefit from repeat measurements, frequent artificial tears, and a treatment regimen of Restasis (cyclosporine A 0.05%, Allergan), Cequa (cyclosporine ophthalmic solution 0.09%, Sun Pharmaceuticals) or Xiidra (lifitegrast 5%, Novartis).24
Patients with a history of refractive surgery often prefer to maintain spectacle independence. Prior vision correction surgery presents two unique challenges. First, increased corneal aberrations after ablative procedures may create further loss of contrast sensitivity, decreased best-corrected visual acuity (BCVA) and increased dysphotopsia. Second, refractive outcomes are less predictable, with a higher incidence of residual refractive error. A second refractive procedure can be offered as an enhancement if residual refractive error is significant after cataract surgery. Patients should be thoroughly educated on risks in these cases, though typically risks are lower than that of a lens exchange.
During preoperative evaluation, pay special attention to pupil size and angle kappa. Eyes with large pupils may not get as much benefit from some pupil-dependent premium lenses. With large angle kappas, the light rays from an object fall at a greater distance from the fovea, resulting in glare or halos.25
Carefully evaluate eyes with any macular or optic nerve pathology before deciding if presbyopia-correcting IOLs are appropriate. Premium lenses are generally contraindicated in the severe progressive pathologies. Monofocal IOLs may be better for patients with glaucoma, macular degeneration or other diseases to preserve BCVA.
Amblyopia should also be ruled out with potential acuity testing.
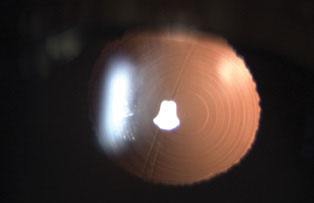
 |
| The ring pattern indicates that the patient has a multifocal IOL, though it may be difficult to identify the specific lens based on appearance. Click image to enlarge. |
Step Three: Anticipate Postoperative Problems
The best way to avoid premium IOL pitfalls is by predicting and preventing them prior to surgery. However, even with careful planning, patients can end up dissatisfied. The most common cause of dissatisfaction in patients with multifocal implants is residual refractive error, followed by dry eye, glare and halos.26-28
Residual refractive error may be addressed with laser vision correction; however, it is vital to allow for adequate healing and stabilization of corneal topography prior to any refractive surgery. Refractive surprises may occur unpredictably, but are more likely in eyes with particularly short or long axial lengths, a history of previous refractive surgery or both. Surgical practices may include laser vision correction enhancement in their premium IOL packages in case of a “refractive surprise.” It may be prudent to discuss such policies with the surgical clinic to prepare your patients for such cases. Patients usually do well with premium IOLs once the residual refractive error is corrected.
If residual astigmatism is caused by rotation of toric multifocals off the intended axis, the patient should be sent back to the surgeon and the lens should be rotated into the correct position within the first few weeks after surgery.
In cases of early posterior capsular opacification, YAG capsulotomy may be needed, but it would be wise to wait at least three months to make sure the patient adapts well. If there is any chance that a lens exchange may be done, YAG capsulotomy should be delayed, as an open posterior capsule makes the exchange more difficult.
Contrast sensitivity may be significantly affected due to division of light that occurs with the multifocal design.6 Apart from exchanging the IOL for a monofocal implant, nothing can be done to correct this.
One of the most common concerns reported by patients after multifocal implantation is nighttime dysphotopsia. This can occur even with monofocal IOL, but is more prevalent with premium lens designs.6 The most important aid in managing these patients is time. Because multifocal IOLs divide incident light into multiple focal points, your patient’s brain must adjust to process several images simultaneously. This process of neuroadaptation can take time and cause frustration, so patients must be prepared for it going in.6
Neuroplasticity is the ability of the brain to reorganize its function and structure in response to environmental changes.29 Brain activity can be examined with functional magnetic resonance imaging (MRI). Using this technology, we know that patients who recently underwent multifocal IOL implantation have increased activity in their cortical areas dedicated to visual attention and effortful action, procedural learning, cognitive control and goal-oriented behavior.30 Researchers also note a correlation between level of dysphotopsia symptoms and level of activity in the top-down attentional network in the parietal and frontal lobes as well as cingulate cortex and caudate nucleus.30 Investigators also show that when functional MRI is repeated six months after multifocal IOL implantation, the regions of the brain associated with attentional network and effort are less activated.31 This decrease in brain activation correlates with improved dysphotopsia symptoms at six months.31 Since no measurable difference exists between optical parameters over the same time period, researchers believe neuroadaptation is the only explanation for such changes.32-33
Neuroadaptation is highly dependent on the individual. Personality traits to watch for include compulsive checking, orderliness, competence and dutifulness. Research suggests people who possess those qualities are more likely to experience glare and halos, and may fail to neuroadapt.34
Neuroadaptation plays an important role in multifocal outcomes, especially positive dysphotopsia. No effective treatment for these symptoms is available. Four to 12% of cases in which bothersome glare, halos or starbursts are present are due to an IOL defect.31 In cases when patients still report bothersome dysphotopsia a month after surgery, doctors should offer reassurance and encouragement and allow for neuroadaptation to take over. If a patient is still bothered by dysphotopsia three to four months after surgery, or if their quality of life is significantly affected, they may consider switching out the premium lens for a monofocal implant.
Lens exchange, of course, is a last resort. Exchanging an IOL involves additional risks and costs to the patient. It helps if patients are aware of the possibility of a lens exchange prior to the initial surgery. If it does come down to this, it is best to do the exchange within the six months. Better understanding of neuroadaptive mechanism in the future may help optometrists and ophthalmologists better manage dysphotopsia and improve multifocal outcomes.
 |
| In most cases, multifocal IOL rings are visible even through an undilated pupil, as seen here. Click image to enlarge. |
Step Four: Consider Adjustable IOLs
Given that residual refractive error is the most common reason for intraocular lens exchange, any technology that can improve accuracy is worth considering. In 2017, The RxLAL, (light adjustable lens, RxSight) was FDA approved and boasts the ability to non-surgically refine unexpected postoperative refractive error.35,36 Corrective treatments are conducted using the company’s light delivery device (LDD) and can alter up to 2D of spherical power or between 0.75D to 2D of astigmatism correction.36
The optics of the lens change via UV-sensitive macromers that, when stimulated, polymerize and thicken the intraocular lens where needed. For example, if a patient ended up hyperopic post-cataract surgery, the LDD would be programmed to photopolymerize the central portion of the lens, thereby thickening and increasing the power of the lens. For myopic surprises, the UV light would be concentrated in the periphery instead.
Several adjustments can be made once the patient is three weeks out of surgery to customize their vision. The flexibility of this lens offers the ability to optimize monovision endpoints and to minimize refractive surprise in those with history of laser vision correction.
Contraindications for this lens include conditions and medications that could cause sensitivity or adverse reactions to UV exposure. A careful review of the patient’s systemic health should be conducted.
In the future, new lenses and devices may even allow patients to temporarily trial presbyopia-correcting optics with the ease of a UV treatment to revert back to single-focus properties if they are unable to adapt. Two companies—Perfect Lens and Clerio Vision—are currently developing such technologies. The ability to perform a noninvasive postoperative adjustment to overcome refractive surprises would be a major paradigm shift in cataract surgery. This would present more options to the patients postoperatively and greatly improve accuracy of visual outcomes.
Patients increasingly desire more spectacle independence after cataract surgery. Multifocal IOLs can provide excellent uncorrected vision across a range of distances, but may cause dysphotopsia and patient dissatisfaction. Careful selection of candidates, thorough preoperative education and high surgical skill all greatly increase chances of success with multifocals. Personality assessment should be included in the patient’s regular preoperative ocular exam. The role of neuroadaptation cannot be underestimated, and the vast majority of post-op concerns resolve with time. Laser vision correction or IOL exchange remain an option when patients are dissatisfied with significant residual refractive error or bothersome dysphotopsia.
With adjustable IOLs beginning to enter the market, it may soon be common to improve refractive outcomes postoperatively. But for now, optometrists must perfect their preoperative examination and patient education protocol to continue increasing their surgical comanagement role.
Dr. Sukhovolskiy is a staff optometrist at Pacific Cataract and Laser Institute, practicing in Tacoma, WA.
Dr. Roan is a staff optometrists at Pacific Cataract & Laser Institute, in Bellevue, WA.
1. Khandelwal S, Jun J, Mak S, et al. Effectiveness of multifocal and monofocal intraocular lenses for cataract surgery and lens replacement: a systematic review and meta-analysis. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2019;257(1):863-875. 2. Mendicute J, Kapp A, Levy P, et al. Evaluation of visual outcomes and patient satisfaction after implantation of a diffractive trifocal intraocular lens. J Cataract Refract Surg. 2016;42(2):203-210. 3. Yamauchi T, Tabuchi H, Takase K, et al. Comparison of visual performance of multifocal intraocular lenses with same material monofocal intraocular lenses. PLOS ONE. 2013;8(6):e68236. 4. De Silva SR, Evans JR, Kirthi V, et al. Multifocal versus monofocal intraocular lenses after cataract extraction. Cochrane Database Syst Rev. 2016;12:CD003169. 5. Rosen E, Alio JL, Dick H, et al. Efficacy and safety of multifocal intraocular lenses following cataract and refractive lens exchange. Metaanalysis of peer-reviewd publications. J Cataract Refract Surg. 2016;42(2):310-28. 6. Alio J, Plaza Puche A, Fernandez-Buenaga R, et al. Multifocal intraocular lenses: an overview. Surv Ophthalmol. 2017;62(5):611-634. 7. Sachdev G, Sachdev M. Optimizing outcomes with multifocal intraocular lenses. Indian J Ophthalmol. 2017;65(12):1294-300. 8. Sieburth R, Chen M. Intraocular lens correction of presbyopia. Taiwan J Opthalmol. 2019;9(1):4-17. 9. Xu X, Zhu M, Zou HD. Refractive versus diffractive multifocal intraocular lenses in cataract surgery: a meta-analysis of randomized controlled trials. J Refract Surg. 2014;30(9):634-44. 10. Weghaupt H, Pieh S, Skorpik C. Comparison of pseudoaccommodation and visual quality between a diffractive and refractive multifocal intraocular lens. J Cataract Refract Surg. 1998;24(5):663-5. 11. Yang J, Liu Q, Li J, Qin L. Comparison of visual outcomes with implantation of trifocal versus bifocal intraocular lens after phacoemulsification: A meta-analysis. Int J Ophthalmol. 2018;11(3):484-92. 12. Xu Z, Cao D, Chen X, et al. Comparison of clinical performance between trifocal and bifocal intraocular lenses: A meta-analysis. PLoS One. 2017;12(10):e0186522. 13. Shen Z, Lin Y, Zhu Y, et al. Clinical comparison of patient outcomes following implantation of trifocal and bifocal intraocular lenses: A systematic review and meta-analysis. Sci Rep. 2017;7:45337. 14. Sudhir RR, Dey A, Bhattacharrya S, Bahulayan A. Acrysoft IQ PanOptix intraocular lens versus extended depth of focus intraocular lens and trifocal intraocular lens: a clinical overview. Asia Pac J Ophthalmol (Phila). 2019 Jul-Aug; 8(4): 335–49. 15. Pedrotti E, Carones F, Aiello F, et al. Comparative analysis of visual outcomes with 4 intraocular lenses: Monofocal, multifocal, and extended range of vision. J Cataract Refract Surg. 2018;44(2):156-167. 16. Savini G, Schiano-Lomoriello D, Balducci N, Barboni P. Visual performance of a new extended depth-of-focus intraocular lense compared to a distance-dominant diffractive multifocal intraocular lens. J Refract Surg. 2018;34(4):228-35. 17. Cochener B, Boutillier G, Lamard M, Auberger-Zagnoli C. A comparative evaluation of a new generation of diffractive trifocal and extended depth of focus intraocular lenses. J Refract Surg. 2018;34(8):507-14. 18. Monaco G, Gari M, Censo FD, et al. Visual performance after bilateral implantation of 2 new presbyopia-correcting intraocular lenses: Trifocal versus extended range of vision. J Cataract Refract Surg. 2017;43(6):737-47. 19. de Medeiros AL, de Araujo Rolim AG, Pimenta Motta AF, et al. Comparison of visual outcomes after bilateral implantation of a diffractive trifocal intraocular lens and blended implantation of an extended depth of focus intraocular lens with a diffractive bifocal intraocular lens. Clin Ophthalmol 2017;11(10):1911-6. 20. Ferrer-Blasco T, Montés-Micó R, Peixoto-de-Matos SC, et al. Prevalence of corneal astigmatism before cataract surgery. J Cataract Refract Surg. 2009;35(1):70–75. 21. Khan MI, Muhtaseb M. Prevalence of corneal astigmatism in patients having routine cataract surgery at a teaching hospital in the United Kingdom. J Cataract Refract Surg. 2011;37(10):1751–1755. 22. Michelitsch M, Ardjomand N, Vidic B, et al. Prevalence and age-related changes of corneal astigmatism in patients before cataract surgery. Ophthalmologe. 2017;114(3):247–51. 23. Pepose JS. Maximizing satisfaction with presbyopia-correcting intraocular lenses: The missing links. Am J Ophthalmol. 2008;146(5):641-8. 24. Donnenfield ED, Solomon R, Roberts CW, et al. Cyclosporine 0.05% to improve visual outcomes after multifocal intraocular lens implantation. J Cataract Refract Surg. 2010;36(7):1095-100. 25. Karhanova M, Maresova K, Pluhacek F, et al. The importance of angle kappa for centration of multifocal intraocular lenses. Cesk Slov Oftalmol. 2013;69(2):64-8. 26. Gibbons A, Ali TK, Waren DP, Donaldson KE. Causes and correction of dissatisfaction after implantation of presbyopia-correcting intraocular lenses. Clin Ophthalmol. 2016;10(10):1965-70. 27. Mamalis N, Brubaker J, Davis D, et al. Complications of foldable intraocular lenses requiring explantation or secondary intervention – 2007 survey update. J Cataract Refract Surg. 2008;34(9):1584-91. 28. Kamiya K, Hayashi K, Shimizu K, et a. Multifocal intraocular lens explantation: A case series of 50 eyes. Am J Ophthalmol. 2014;158(2):215-20.el. 29. Nelson C. Neural plasticity and human development: the role of early experience in sculpting memory systems. Dev Sci. 2000;3(12):115-36. 30. Rosa A, Miranda A, Patricio M, et al. Functional magnetic resonance imaging to assess the neurobehavioral impact of dysphotopsia with multifocal intraocular lenses. Ophthalmol. 2017;124(9):1280-9. 31. Rosa A, Miranda A, Patricio MM, et al. Functional magnetic resonance imaging to assess neuroadaptation to multifocal intraocular lenses. J Cataract Refract Surg. 2017;43(10):1287-96. 32. Wilkins M, Allan B, Rubin G, et al. Randomized trial of multifocal intraocular lenses versus monovision after bilateral cataract surgery. Ophthalmol. 2013;120(12):2449-2455.e1 33. Kinard K, Jarstad A, Olson RJ. Correlation of visual quality with satisfaction and function in a normal cohort of pseudophakic patients. J Cataract Reftract Surg. 2013;39(4):590-7. 34. Mester U, Vaterrodt T, Goes F, et al. Impact of personality characteristics on patient satisfaction after multifocal intraocular lens implantation: results from the “happy patient study.” J Refract Surg. 2014;30(10):674-8. 35. Bethke W. RxLAL: Say Goodbye to Postop Surprise? Rev Ophthamol. 2018;25(1):12-5;66 36. FDA.gov. LAL Summary of Safety and Effectiveness. www.accessdata.fda.gov/cdrh_docs/pdf16/P160055B.pdf. November 22, 2017. Accessed February 15, 2020. 36. van Asten F, Michels CTJ, Hoyng CB, et al. The cost-effectiveness of bevacizumab, ranibizumab and aflibercept for the treatment of age-related macular degeneration: A cost-effectiveness analysis from a societal perspective. PLOS ONE 2018;13(5):1-14. |

