Central serous chorioretinopathy (CSCR) is a common retinal disorder that results in vision loss and alteration of visual function.1 It is considered a pachychoroid disease, a category of diseases that includes polypoidal choroidal vasculopathy, pachychoroid neovasculopathy and pachychoroid pigment epitheliopathy.2 Despite CSCR’s prevalence as the fourth most common non-surgical retinopathy behind macular degeneration, diabetic retinopathy and branch retinal vein occlusion, there is still no standard treatment regimen for the condition.1
A variety of therapies and potential lifestyle modifications have been explored with different outcomes, including focal photocoagulation, photodynamic therapy (PDT) with verteporfin, intravitreal anti-vascular endothelial growth factor (anti-VEGF) agents and several classes of oral and topical medications. More recently, investigation into mineralocorticoid receptor antagonist (MRA) drugs has produced hopeful results. With these medications in mind as an early intervention option, this article proposes a potential treatment scheme for patients diagnosed with CSCR.
 |
|
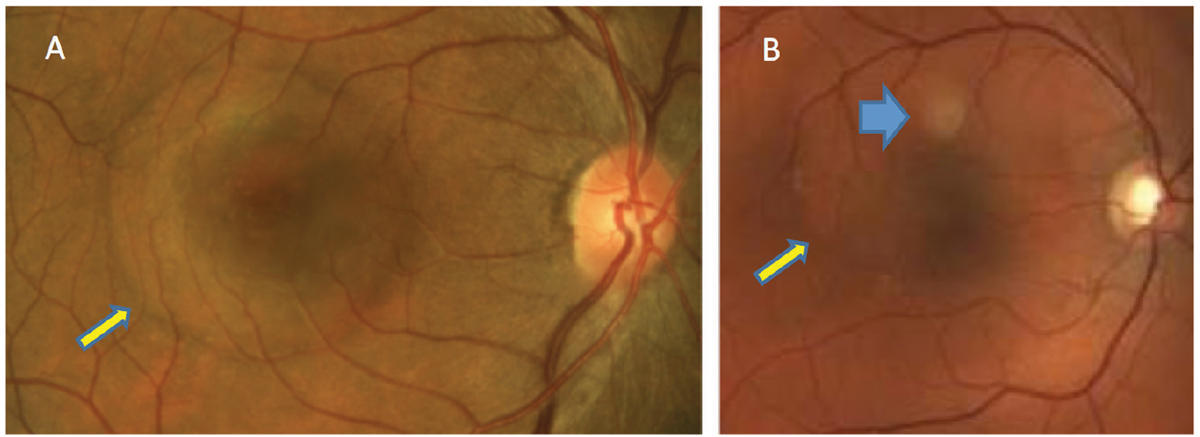
Fig. 1. In patients with acute CSCR, serous retinal detachment can be seen (yellow arrows). PED is not clinically detected in patient A but can be seen in patient B (blue). Click image to enlarge. |
Background
CSCR is typically found in men and women of all ethnic backgrounds after the third decade of life. The common acute presentation occurs most often in middle-aged Caucasian males.3,4 The incidence rate is approximately 10 men and two women per 100,000 people.3,4
The most common risk factors for the condition include “type A” personality, pregnancy and systemic use of corticosteroids and adrenergic receptor inhibitors. Other identified potential risk factors include systemic hypertension, Helicobacter pylori gastrointestinal disease, testosterone supplementation and sleep apnea.5-7
Clinical findings depend on the duration of CSCR, with findings associated with the condition detectable during retinal examination. In acute cases, patients will present with varying degrees of visual disturbance. Usually, a round serous retinal detachment (SRD) that varies in size is noted within the posterior pole. A pigment epithelial detachment (PED) may or may not be detectable clinically (Figure 1).
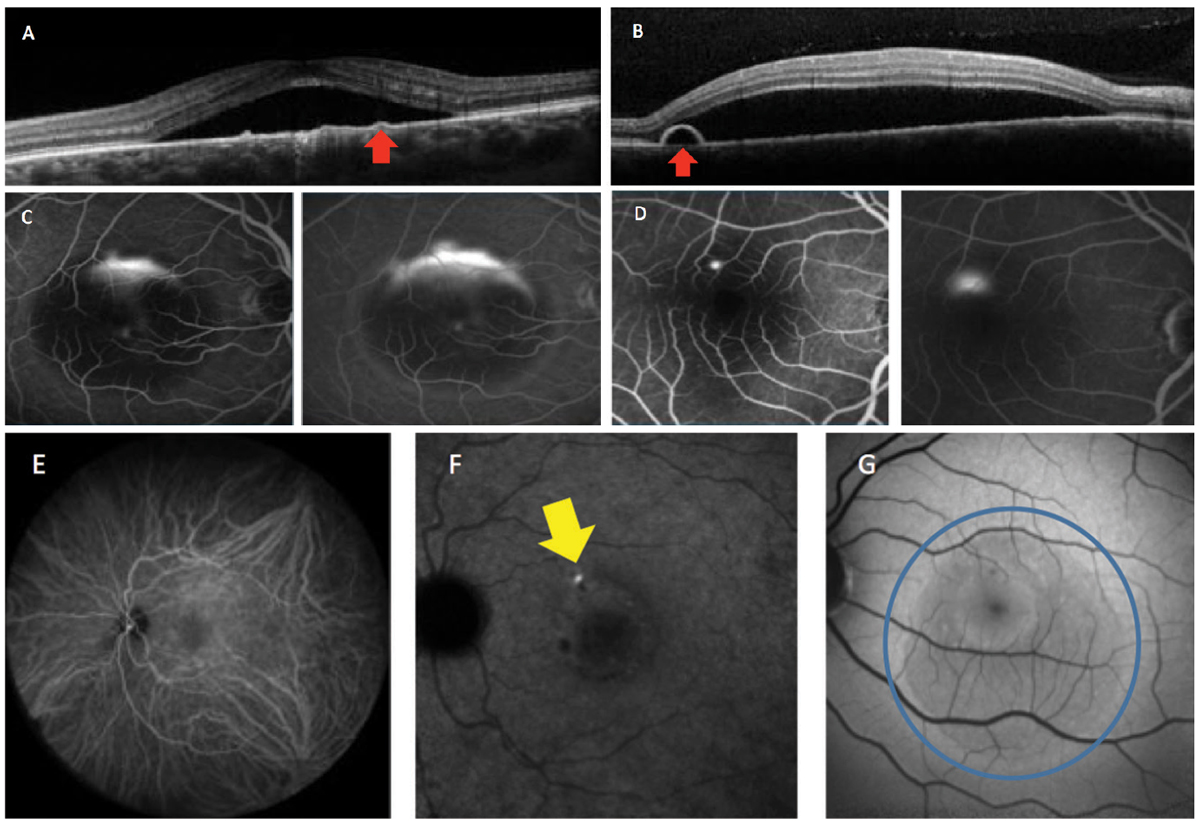
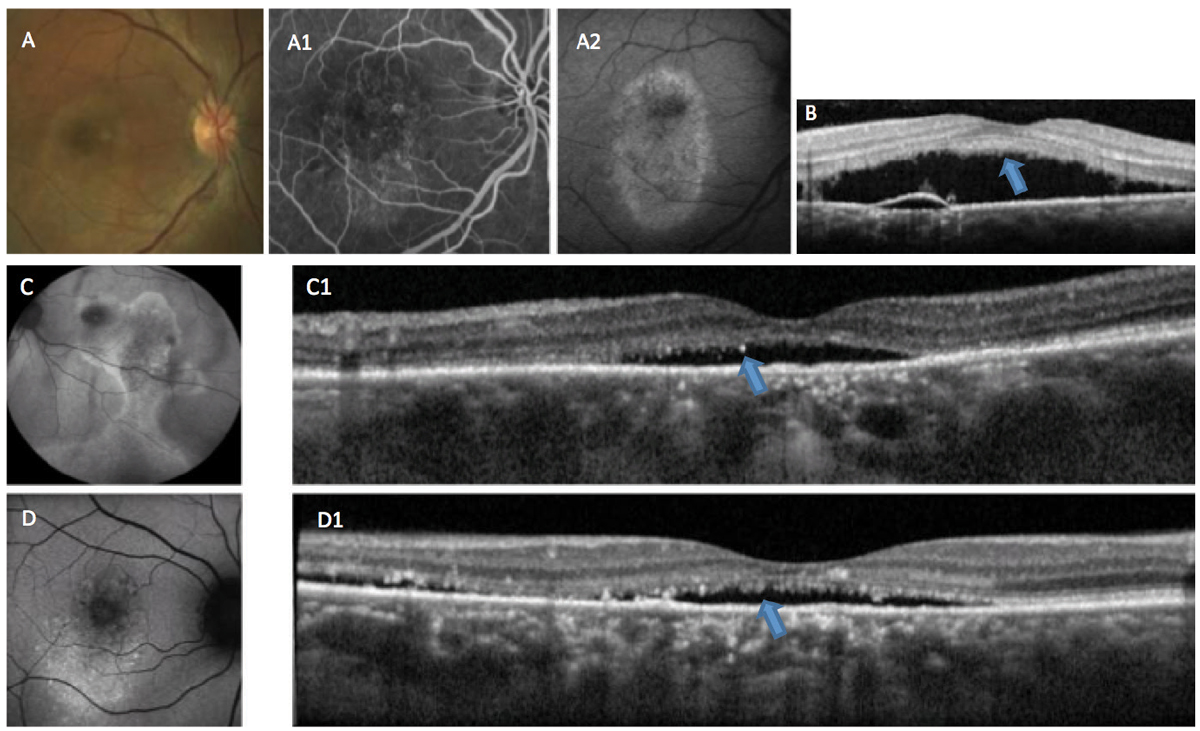
Ancillary tests, such as spectral-domain OCT, fluorescein angiography (FA), indocyanine green (ICGA) angiography, OCT angiography and fundus autofluorescence (FAF), are helpful to detect the various associated features of CSCR.8-10 These imaging modalities are not only useful in proper diagnosis of the condition, but also in determination of staging (acute or chronic) (Figures 2 and 3).
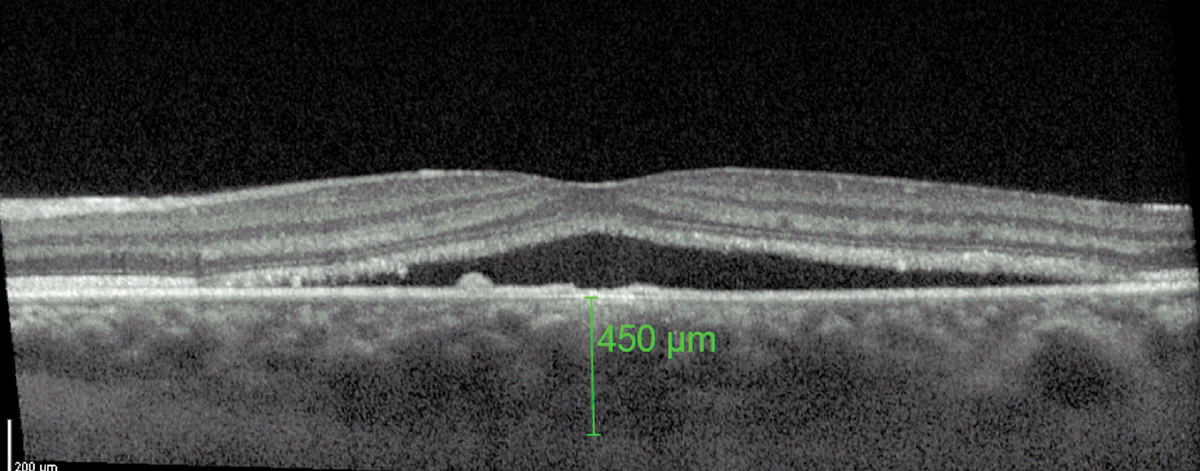
On OCT, a common feature of CSCR is a greater-than-average choroidal thickness (Figure 4).11 One study reported that patients with CSCR had a mean subfoveal choroidal thickness of 475±138µm compared with 372±120µm in healthy patients.11
Although most acute cases spontaneously resolve with minimal to no long-term vision loss, persistent or chronic CSCR can result in permanent alteration of the retinal pigment epithelium (RPE) and retinal photoreceptors (RPs). This can consequently cause permanent vision loss. Therefore, in chronic cases lasting longer than four months, therapy should be targeted to lessen the risk of permanent functional visual loss.12-14
The exact pathophysiology of CSCR is not well known, thus a gold standard of care has not yet been established.6 Investigational treatments have been based on the potential pathophysiological and etiologic factors.
 |
|
Fig. 2. This patient has acute CSCR. OCT imaging demonstrates neurosensory serous retinal detachments, with (A) showing a flat PED while (B) shows a bulging PED (red arrows). Fluorescein angiography (C) shows the classic “smokestack” pattern of leakage and (D) the more common “inkblot” pattern. Indocyanine green angiography shows the mid (E) and late (F) phases. Active leakage can be seen in the late phase (yellow arrow). Fundus autofluorescence (G) shows hypo-autofluorescence in acute or early cases (blue circle). Click image to enlarge. |
Treatment Strategies
Optometry, in collaboration with retina surgeons, has taken several different routes in treating CSCR and establishing a consistent treatment protocol.
Thermal photocoagulation with argon laser. This modality—the oldest form of treatment—is based on mechanically treating the PED or RPE defect, which is the assumed defective, “leaky” area causing SRD. This area can be detected by FA and ICGA.15,16 Damage to this area can terminate the active leakage and result in faster resolution of the SRD; however, due to collateral damage caused by the thermal laser, this therapy cannot be applied to diffuse areas or regions that are too close to the fovea. Risks include formation of scotoma, formation of laser scars and potentially development of choroidal neovascular membranes.15
PDT with verteporfin. This treatment addresses the notion that CSCR is a condition of leaky, abnormal choroidal vessels, specifically within the choriocapillaris. Application of PDT laser is thought to lead to a cascade of events causing reduced choriocapillaris congestion and vascular remolding, resulting in decreased choroidal permeability.17 PDT laser can be applied to broader and more central areas than focal argon laser, and is often guided by FA or ICGA to target leaky areas. The initial short-term case series followed standard PDT protocol.18 Since then, investigation into modified strategies, such as half-dose PDT and half-fluence PDT, have shown good results with a better risk profile. Unfortunately, a consensus still hasn’t been reached on PDT protocol in CSCR patients.19 Clinically, PDT laser availability and cost coverage are practical barriers to the treatment.
 |
|
Fig. 3. This patient is suffering from chronic CSCR. In (A), RPE changes can be seen in the macula. Fluorescein angiography (A1) shows staining of the altered RPE, and fundus autofluorescense (A2) shows hyper-autofluorescence indicative of RPE damage. OCT (B) shows accumulation of RP outer segment shedding (“shaggy” photoreceptors), a phenomenon associated with choroidal melanomas (blue arrow). Autofluorescence (C and D) in other cases shows a variable mix of autofluorescence associated with chronicity and recurrence. OCT (C1 and D1) shows adverse alteration of the outer retina (blue arrows). Click image to enlarge. |
Intravitreal injection of anti-VEGF. This route has also been explored as a possible therapeutic option for CSCR, as a chemical method of altering choriocapillaris hyper-permeability. Multiple small case series show potential benefit with anti-VEGF treatment.20-24 However, skepticism of the treatment exists, as CSCR does not seem to be VEGF-driven and increased levels of VEGF are not found in those with the condition. A recent meta-analysis could not verify a positive effect of intravitreal bevacizumab in patients with CSCR.25 It is of importance to note that patients with CSCR are at increased risk of developing choroidal neovascularization, of which anti-VEGF is the treatment of choice.1
Topical meds. Various topical agents have been investigated, including non-steroidal anti-inflammatory drugs (NSAIDs) and carbonic anhydrase inhibitors (CAIs). Multiple small-cohort retrospective trials and case studies report increased rates of subretinal fluid (SRF) reabsorption in acute CSCR with use of topical NSAIDs.26-30
Critics of this treatment modality suggest a possible placebo effect, especially in those with a type A personality, and dispute a proven inflammatory etiology of CSCR.31 A prospective trial investigated topical dorzolamide in patients with chronic CSCR and found a greater improvement in central macular thickness at three months compared with controls.32
Systemic meds. Another known driving factor behind this condition is increased levels of corticosteroids, as a high incidence of CSCR is associated with systemic steroid use, pregnancy and potentially episodes of high stress in susceptible individuals. A number of off-label systemic medications have been explored with this mechanism in mind, showing a range of therapeutic efficacy. These therapeutic agents include MRAs, rifampin and CAIs such as acetazolamide.33-37 At present, the most promising drug category for the treatment of CSCR is MRAs.
MRAs. A number of reports have shown the benefit of two steroidal compound mineralocorticoid receptor antagonists in the treatment of acute and chronic CSCR: spironolactone and eplerenone.38-47
Spironolactone is a non-selective aldosterone (a mineralocorticoid hormone) antagonist and potassium-sparing diuretic indicated for the management of primary hyperaldosteronism and edematous-related conditions, congestive heart failure (CHF), cirrhosis of the liver and nephrotic syndrome. It is also indicated for treatment of essential hypertension, hypokalemia and severe heart failure.
 |
|
Fig. 4. A thicker-than-average choroid is characteristic of CSCR. Click image to enlarge. |
This agent is contraindicated in patients with anuria, acute renal insufficiency, significant impairment of renal excretory function or hyperkalemia. It interacts with angiotensin-converting enzyme inhibitors, alcohol, barbiturates, narcotics, pressor amines and skeletal muscle relaxants.
While spironolactone has a good affinity for the mineralocorticoid receptor, being non-specific it also binds to progesterone receptors, causing dose-dependent hormonal side effects.4
Adverse reactions may include gastric bleeding, nausea/vomiting, diarrhea, gynecomastia, erectile dysfunction, irregular menses, agranulocytosis, hypersensitivity, hyperkalemia, mental dysfunction, headache, drowsiness and renal dysfunction.
Eplerenone, created to lessen the hormonal side effects of spironolactone, is a selective aldosterone antagonist indicated for improving survival of stable patients with left ventricular systolic dysfunction, CHF after myocardial infarctions or hypertension as a mono or combination therapy. While the side effects of eplerenone are favorable compared to those of spironolactone, it is a much less potent MRA. Spironolactone has a 40-fold higher binding affinity to the mineralocorticoid receptor in comparison with eplerenone.4
For a complete list of contraindications, precautions and adverse reactions associated with both MRAs, refer to the prescribing information that accompanies each. In addition to obtaining a complete history of illnesses, medications and allergies, prescribers should consult the patient’s primary care physician and obtain baseline serum potassium levels before administering these medications.
The usual off-label administered dose for treatment of CSCR with spironolactone is 50mg daily and 25mg to 50mg daily for eplerenone until resolution of SRF.38-47 At this dose, spironolactone can reduce both SRF and subfoveal choroidal thickness compared with placebo in patients with persistent SRF due to CSCR.38-40 Eplerenone has also resulted in significant structural and functional improvement in patients suffering from chronic CSCR.41-47
Proposed strategy. Due to the variability of treatment response, there is currently no standard of care in managing CSCR patients. As evidenced by combined treatment strategies and ongoing investigation, no single therapy works sufficiently in all patients. As such, treating patients with CSCR is a highly individualized process with many different considerations and options to take into account. Weighing the risk vs. the benefit of therapy must always be at the forefront of the physician’s treatment approach. A tentative treatment protocol, incorporating MRA as an early treatment option, involves the following:
- Obtain case history.
- Alter any modifiable risk factors.
- Determine if condition is chronic or acute.
- If acute, monitor. If the case doesn’t resolve in four months, proceed to chronic staging.
- If chronic, initiate treatment with MRA if not contraindicated and obtain baseline serum potassium levels. Monitor for one month.
- If improvement is observed, continue therapy until SRD resolves before discontinuing and monitoring.
- If improvement is not observed, consider switching to an alternate MRA and obtain FA or ICGA to guide laser treatment.
- In the case of localized, non-central leakage, apply a focal laser.
- In the case of diffuse, central leakage, apply PDT.
- If improvement is observed, monitor and coordinate additional therapy as needed.
- If improvement is not observed, consider treatment with anti-VEGF.
 |
|
Fig. 5. Acute case of CSCR (top). Resolution at one year without treatment (bottom). Click image to enlarge. |
Case Examples
The following cases illustrate the proposed treatment strategy for various clinical scenarios:
Acute. A 46-year-old white male presented with an acute-onset dark spot in his vision OS for 10 days. The patient reported no recent increase in stress and no use of any steroid medications. OCT imaging was consistent with an acute case of CSCR, with an entering central foveal thickness (CFT) of 420µm. His baseline visual acuity was 20/25.
Due to the acute nature of the condition, the patient was monitored without treatment. The one-month follow-up showed significant improvement of CFT. The patient was lost to follow-up at this point but presented one year later with complete resolution of SRF and visual acuity of 20/20 (Figure 5).
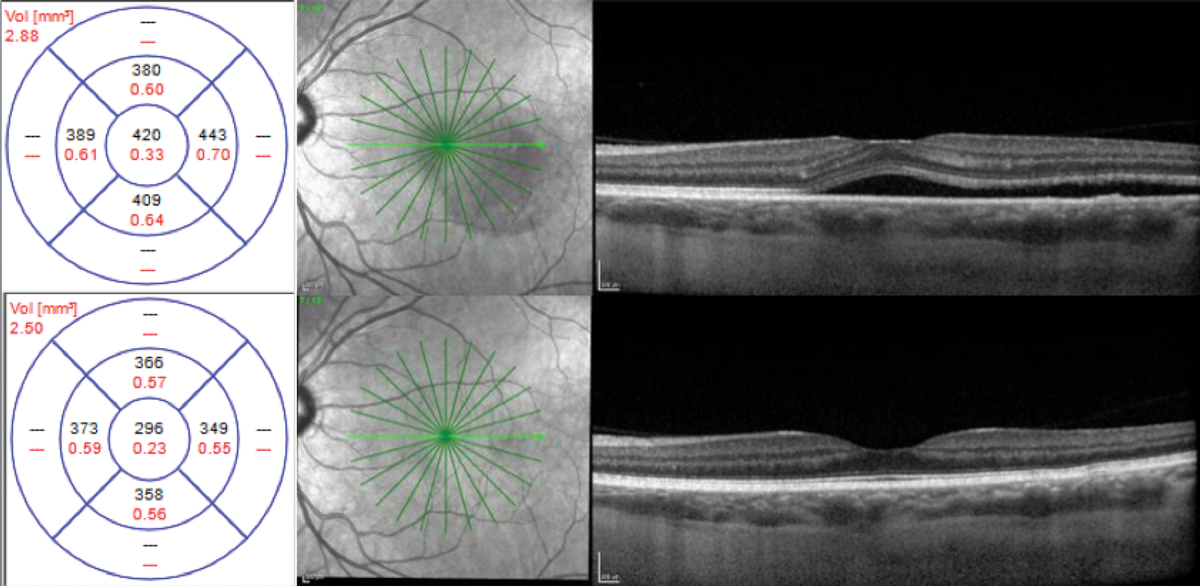
Chronic. A 53-year-old white female presented with a history of chronic CSCR for two years. Her baseline visual acuity was 20/50. Her previous treatment included intravitreal bevacizumab injections and oral acetazolamide without improvement. She was using Flonase (fluticasone, GlaxoSmithKline) daily. At presentation, her CFT was 342µm.
 |
|
Fig. 6. CFT was 342μm at initial presentation (top), 298μm after one month of treatment with eplerenone (middle) and 190μm after two months (bottom). Click image to enlarge. |
With a case history positive for steroid use with Flonase, it was recommended that the patient discontinue the medication immediately. The condition was deemed to be chronic based on the patient’s history and OCT findings. Due to the chronicity of the CSCR, treatment with eplerenone 25mg daily was initiated following a review of risks, benefits and alternatives.
The patient’s CFT at one month was 298µm, and at two months follow-up, it was 190µm with complete resolution of SRF (Figure 6). Upon resolution of SRF, treatment with eplerenone was discontinued. Her final visual acuity was 20/30.
 |
|
Fig. 7. Fundus autofluorescence (top) and OCT (bottom) are both suggestive of chronic disease. Click image to enlarge. |
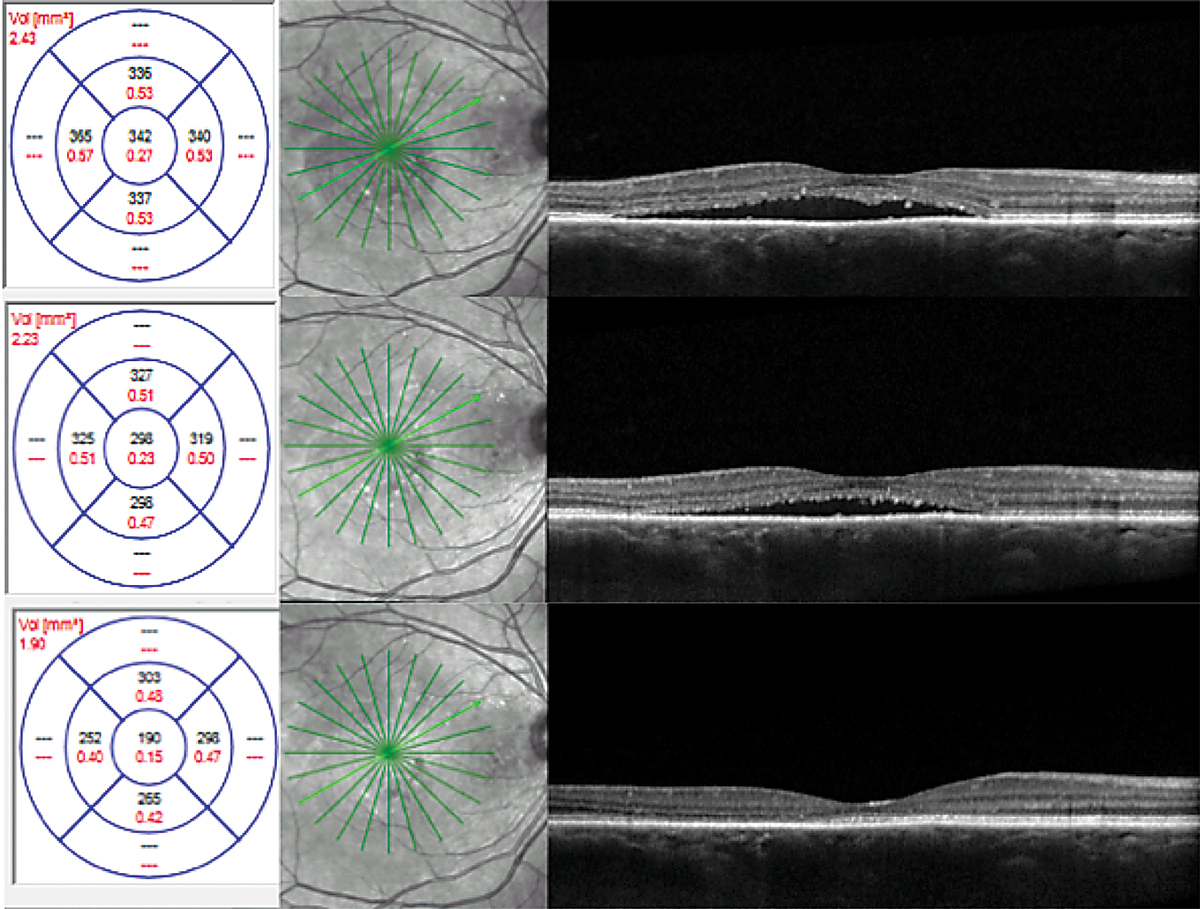
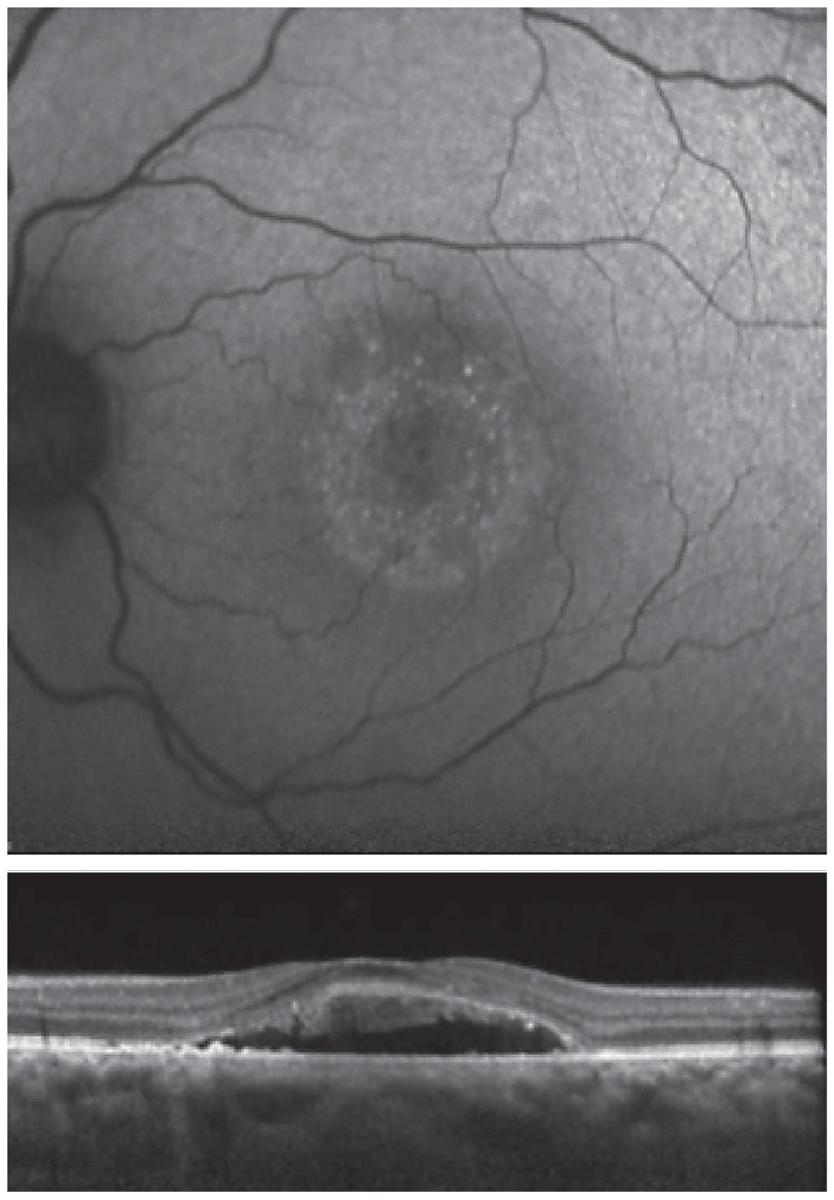
Unknown. A 46-year-old African American female presented with a history of CSCR. She reported symptoms that had been present for four months. Her medical history was positive for high blood pressure, asthma, anemia and kidney disease with a prior kidney transplant. Her medications included amlodipine 5mg BID, calcium carbonate 500mg BID, clonidine 0.1mg TID, aspirin 81mg daily, docusate 100mg BID, dapsone 100mg daily, fluconazole 100mg daily, hydralazine 25mg TID, furosemide 40mg daily, two tablets of magnesium oxide 400mg QID, metoprolol tartrate 25mg BID, mycophenolate sodium 180mg TID, pantoprazole 40mg daily, prednisone 5mg daily, montelukast 10mg daily and tacrolimus 1mg six times daily.
OCT and FAF imaging were both suggestive of chronic CSCR (Figure 7). While the patient’s medical history was positive for steroid use, her options for discontinuation were limited by her prior kidney transplant. Since her condition was chronic, therapy was initiated with oral spironolactone 50mg daily. Due to her extensive medical history, the patient’s nephrologist was consulted prior to initiation of treatment. There was no improvement with spironolactone therapy; therefore, treatment was changed to eplerenone 25mg daily, also without success.
FA and ICGA were performed. Both revealed a focal, non-central area of late leakage (Figure 8). Focal laser was applied to this area. There was significant improvement of serous fluid one month after and complete resolution two months after, with continued improvement in outer retinal anatomy six months post-treatment (Figure 9).
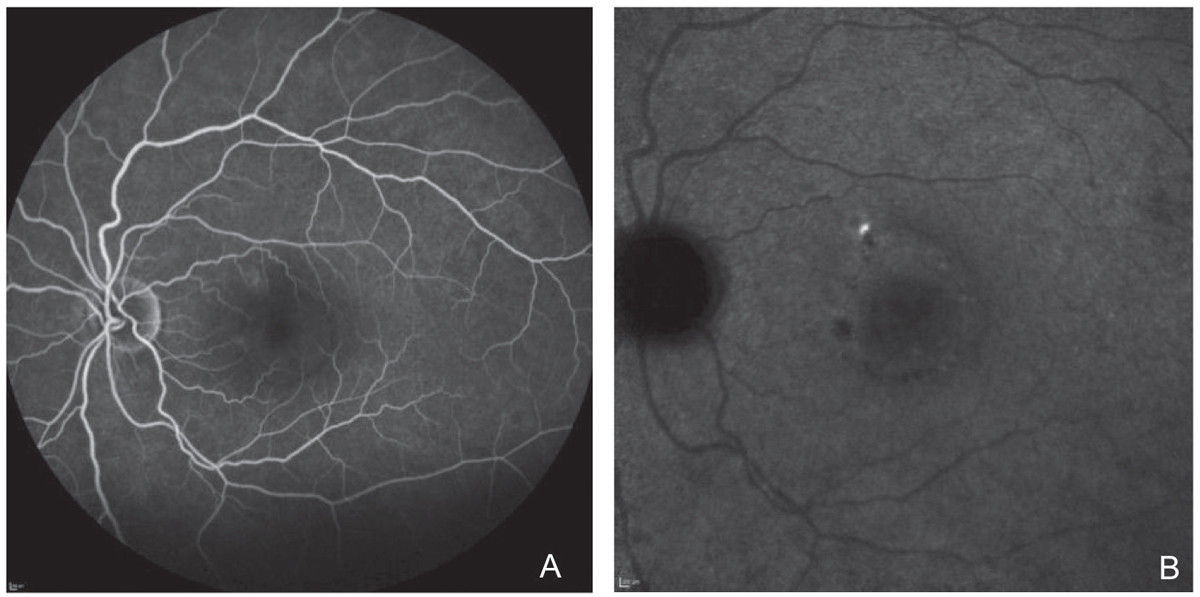 |
| Fig. 8. Localized area of leakage on fluorescein angiography in (A) and ICGA in (B). Click image to enlarge. |
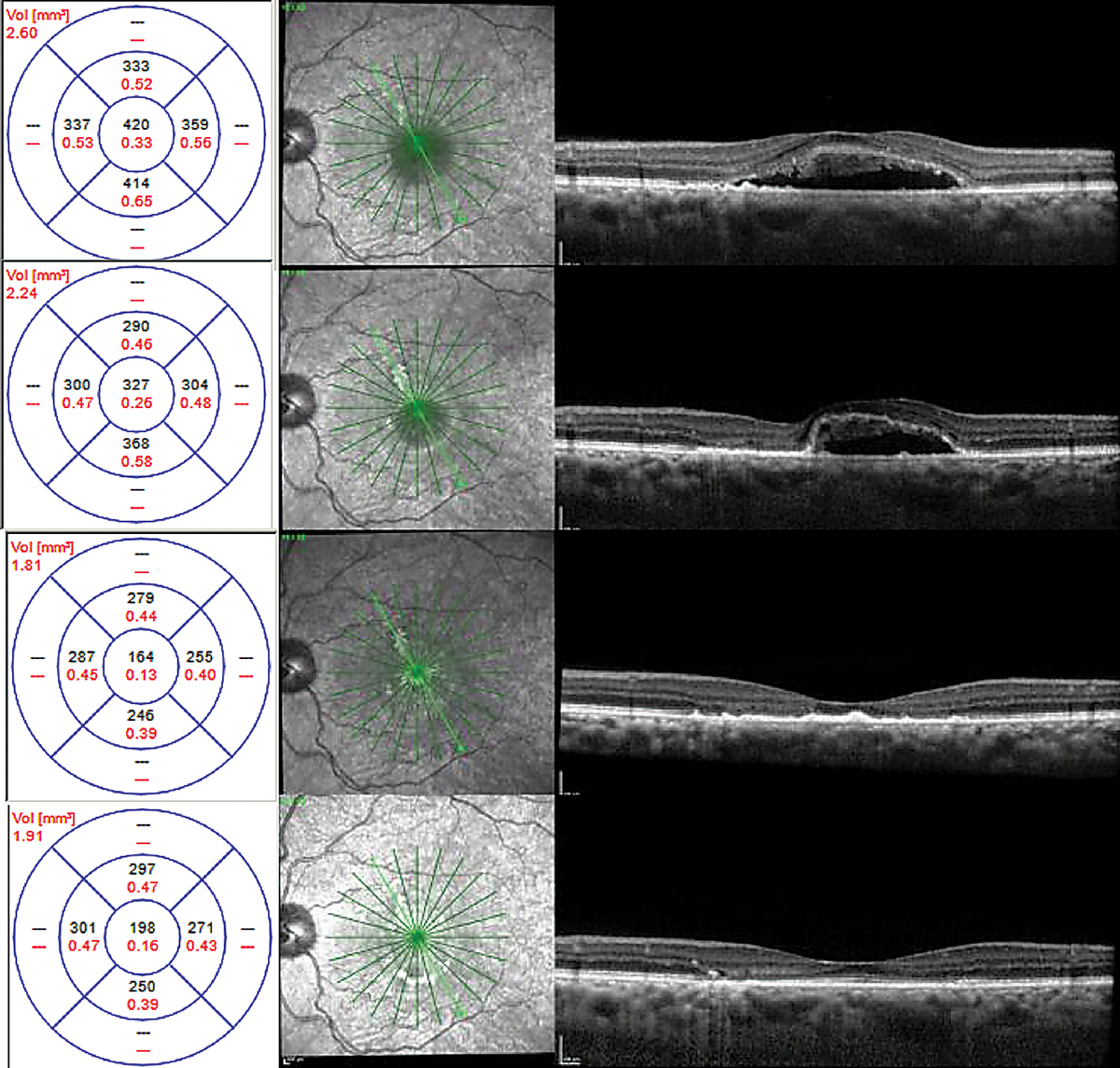 |
| Fig. 9. Chronic CSCR with shaggy photoreceptors at time of laser therapy (top), one month (top middle), two months (bottom middle) and six months (bottom) after focal laser. Click image to enlarge. |
Takeaways
While the management of patients with CSCR remains up to each clinician’s discretion, the proposed treatment protocol takes into account the risk vs. benefit of treatment as well as the currently understood effectiveness of proposed therapies.
While no therapy is without risk, the possibility of improved outcomes in CSCR patients with readily available oral medications makes treatment with MRA a good first-line option in cases of chronic disease persisting longer than four months. Those who do not respond well to MRAs should be considered for additional therapies such as angiographically guided PDT or focal laser, and possibly intravitreal anti-VEGF. Observation of acute cases initially is recommended.
Dr. Rafieetary is a consultative optometrist at the Charles Retina Institute in Germantown, TN. Dr. Haynes is also a consultative optometrist at the Charles Retina Institute and a consulting faculty member at the Southern College of Optometry in Memphis, TN. Dr. Attar is an assistant professor in the Department of Ophthalmology at the University of Mississippi Medical Center. None of the authors have any financial interests to disclose.
1. Wang M, Munch IC, Hasler PW, et al. Central serous chorioretinopathy. Acta Ophthalmol. 2008;86(2):126-45. 2. Demirel S, Değirmenci MFK, Batıoğlu F, et al. Evaluation of the choroidal features in pachychoroid spectrum diseases by optical coherence tomography and optical coherence tomography angiography. Eur J Ophthalmol. 2021;31(1):184-93. 3. Kitzmann AS, Pulido JS, Diehl NN, et al. The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980-2002. Ophthalmology. 2008;115(1):169-73. 4. Spaide RF, Campeas L, Haas A, et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology. 1996;103(12):2070-9. 5. Daruich A, Matet A, Dirani A, et al. Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog Retin Eye Res. 2015;48:82-118. 6. Abouammoh MA. Advances in the treatment of central serous chorioretinopathy. Saudi J Ophthalmol. 2015;29(4):278-86. 7. Nudleman E, Witmer MT, Kiss S, et al. Central serous chorioretinopathy in patients receiving exogenous testosterone therapy. Retina. 2014;34(10):2128-32. 8. Montero JA, Ruiz-Moreno JM. Optical coherence tomography characterisation of idiopathic central serous chorioretinopathy. Br J of Ophthalmol. 2005;89(5):562-4. 9. How ACSW, Koh AHC. Angiographic characteristic of acute central serous chorioretinopathy in an Asian population. Ann Acad Med Singap. 2006;35(2):77-9. 10. Menchini U, Virgili G, Lanzetta P, et al. Indocyanine green angiography in central serous chorioretinopathy. Int Ophthalmol. 1997;21(2):57-69. 11. Kuroda S, Ikuno Y, Yasuno Y, et al. Choroidal thickness in central serous chorioretinopathy. Retina. 2013;33(2):302-8. 12. Ross A, Ross AH, Mohamed Q. Review and update of central serous chorioretinopathy. Curr Opin Ophthalmol. 2011;22(3):166-73. 13. Klein ML, Van Buskirk EM, Friedman E, et al. Experience with nontreatment of central serous choroidopathy. Arch Ophthalmol. 1974;91(4):247-50. 14. Aggio FB, Roisman L, Melo GB, et al. Clinical factors related to visual outcome in central serous chorioretinopathy. Retina. 2010;30(7):1128-34. 15. Robertson DM, Ilstrup D. Direct, indirect, and sham laser photocoagulation in the management of central serous chorioretinopathy. Am J Ophthalmol. 1983;95(4):457-66. 16. Leaver P, Williams C. Argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol. 1979;63(10):674-7. 17. Chan WM, Lam DSC, Lai TYY, et al. Choroidal vascular remodeling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br J Ophthalmol. 2003;87(12):1453-8. 18. Yannuzzi LA, Slakter JS, Gross NE, et al. Indocyanine green angiography-guided photodynamic therapy for treatment of chronic central serous chorioretinopathy: a pilot study. Retina. 2003;23(3):288-98. 19. Iacono P, Parodi MB, Falcomatà B, et al. Central serous chorioretinopathy treatments: a mini review. Ophthalmic Res. 2015;55(2):76-83. 20. Schaal KB, Hoeh AE, Scheuerle A, et al. Intravitreal bevacizumab for treatment of chronic central serous chorioretinopathy. Eur J Ophthalmol. 2009;19(4):613-7. 21. Mehany SA, Shawkat AM, Sayed MF, et al. Role of Avastin in management of central serous chorioretinopathy. Saudi J Ophthalmol. 2010; 24(3):69-75. 22. Tekin K, Sekeroglu MA, Cankaya AB, et al. Intravitreal bevacizumab and ranibizumab in the treatment of acute central serous chorioretinopathy: a single center retrospective study. Semin Ophthalmol. 2018;33(2):265-70. 23. Park SU, Lee SJ, Kim M. Intravitreal anti-vascular endothelial growth factor versus observation in acute central serous chorioretinopathy: one year results. Korean J Ophthalmol. 2014;28(4):306-13. 24. Kim M, Lee SC, Lee SJ. Intravitreal ranibizumab for acute central serous chorioretinopathy. Ophthalmologica. 2013;229(3):152-7. 25. Chung YR, Seo EJ, Lew HM, et al. Lack of positive effect of intravitreal bevacizumab in central serous chorioretinopathy: meta-analysis and review. Eye (Lond). 2013;27(12):1339-46. 26. Alkin Z, Osmanbasoglu OA, Ozkaya A, et al. Topical nepafenac in treatment of acute central serous chorioretinopathy. Med Hypothesis Discov Innov Ophthalmol. 2013;2(4):96-101. 27. Wuarin R, Kakkassery V, Consigli A, et al. Combined topical anti-inflammatory and oral acetazolamide in the treatment of central serious chorioretinopathy. Optom Vis Sci. 2019;96(7):500-6. 28. Bahadorani S, Maclean K, Wannamaker K, et al. Treatment of central serous chorioretinopathy with topical NSAIDs. Clin Ophthalmol. 2019;13:1543-8. 29. Chong CF, Yang D, Pham TQ, et al. A novel treatment of central serous chorioretinopathy with topical anti-inflammatory therapy. BMJ Case Rep. 2012;2012. 30. An SH, Kwon YH. Effect of a topical nonsteroidal anti-inflammatory agent (0.1 % pranoprofen) on acute central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2016;254(8):1489-96. 31. Kaya A, Aksoy Y, Sevinç MK, et al. Comment on: “Effect of a topical nonsteroidal anti-inflammatory agent (0.1 % pranoprofen) on acute central serous chorioretinopathy.” Graefes Arch Clin Exp Ophthalmol. 2016;254(7):1429. 32. Liew G, Ho IV, Ong S, et al. Efficacy of topical carbonic anhydrase inhibitors in reducing duration of chronic central serous chorioretinopathy. Transl Vis Sci Technol. 2020;9(13):6. 33. Jaisser F, Farman N. Emerging roles of the mineralocorticoid receptor in pathology: toward new paradigms in clinical pharmacology. Pharmacol Rev. 2016;68(1):49-75. 34. Shulman S, Goldberg D, Schwartz R, et al. Oral rifampin treatment for longstanding chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2016;254(1):15-22. 35. Sabouri MR. Evaluation of therapeutic effect of rifampin for acute central serous chorioretinopathy. Bina J Ophthalmol. 2013;19(1):46-51. 36. Witjaksana R, Sumual V. Treatment of central serous chorioretinopathy with carbonic anhydrase inhibitor. Ophthalmologica Indonesiana. 2016;42(2):119-22. 37. Pikkel J, Beiran I, Ophir A, et al. Acetazolamide for central serous retinopathy. Ophthalmology. 2002;109(9):1723-5. 38. Bousquet E, Beydoun T, Rothschild PR, et al. Spironolactone for nonresolving central serous chorioretinopathy: a randomized controlled crossover study. Retina. 2015;35(12):2505-15. 39. Herold TR, Prause K, Wolf A, et al. Spironolactone in the treatment of central serous chorioretinopathy—a case series. Graefes Arch Clin Exp Ophthalmol. 2014;252(12):1985-91. 40. Ryan EH, Pulido CM. Central serous chorioretinopathy treatment with spironolactone: a challenge-rechallenge case. Retin Cases Brief Rep. 2015;9(3):235-8. 41. Sun X, Shuai Y, Fang W, et al. Spironolactone versus observation in the treatment of acute central serous chorioretinopathy. Br J Ophthalmol. 2018;102(8):1060-5. 42. Cakir B, Fischer F, Ehlken C, et al. Clinical experience with eplerenone to treat chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2016;254(11):2151-7. 43. Breukink MB, den Hollander AI, Keunen JEE, et al. The use of eplerenone in therapy-resistant chronic serous chorioretinopathy. Acta Ophthalmol. 2014;92(6):e488-90. 44. Singh RP, Sears JE, Bedi R, et al. Oral eplerenone for the management of chronic central serous chorioretinopathy. Int J Ophthalmol. 2015;8(2):310-4. 45. Cioboata M, Alexandrescu C, Hopinca CA, et al. A new treatment approach—eplerenone—in central serous chorioretinopathy—case report. J Med Life. 2016;9(1):92-4. 46. Rahimy E, Pitcher JD, Hsu J, et al. A randomized double-blind placebo-control pilot study of eplerenone for the treatment of central serous chorioretinopathy. Retina. 2018;38(5):962-9. 47. Schwartz R, Habot-Wilner Z, Martinez MR, et al. Eplerenone for chronic central serous chorioretinopathy—a randomized controlled prospective study. Acta Ophthalmol. 2017;95(7):e610-8. |

