 |  |
Minimally invasive glaucoma surgeries (MIGS) have been revolutionary in the treatment of glaucoma. Although drops have traditionally been the mainstay of care, many eyecare providers are seeking other procedural and surgical options to lower and control intraocular pressure (IOP). Essentially, these alternate treatment options are termed “interventional glaucoma,” which is a mindset on how we can proactively approach glaucoma treatment while minimizing compliance issues and side effects and improving patients’ quality of life. From selective laser trabeculoplasty to intracameral injections, MIGS and sustained-release therapy, these treatments all play a larger role in glaucoma management.
We will discuss one of the latest stent additions to keep on your radar—iStent Infinite (Glaukos)—and a new sustained-release drug implant, the iDose TR (Glaukos).
iStent Infinite
Granted clearance by the FDA last year, this device is the first standalone implantable device for patients with primary open-angle glaucoma where previous medical and surgical treatments have failed. These patients are those who have had previous glaucoma filtering surgeries or cilioablative procedures without success.
So, what’s the difference with iStent Infinite? Instead of using two heparin-coated titanium stents, like the iStent inject W (Glaukos), iStent Infinite includes three stents preloaded into an auto-injector system. Stents are inserted along approximately six clock hours around Schlemm’s canal and are designed to lower IOP by restoring the natural physiological outflow of aqueous humor.
In a recent study, 76.1% of patients met the responder endpoint of >20% mean diurnal IOP reduction at 12 months. For patients on the same or fewer medication(s) as baseline, 53% achieved ≥30% mean diurnal IOP reduction without surgical interventions/other events. There were no explants, infections or device-related interventions or hypotony.1
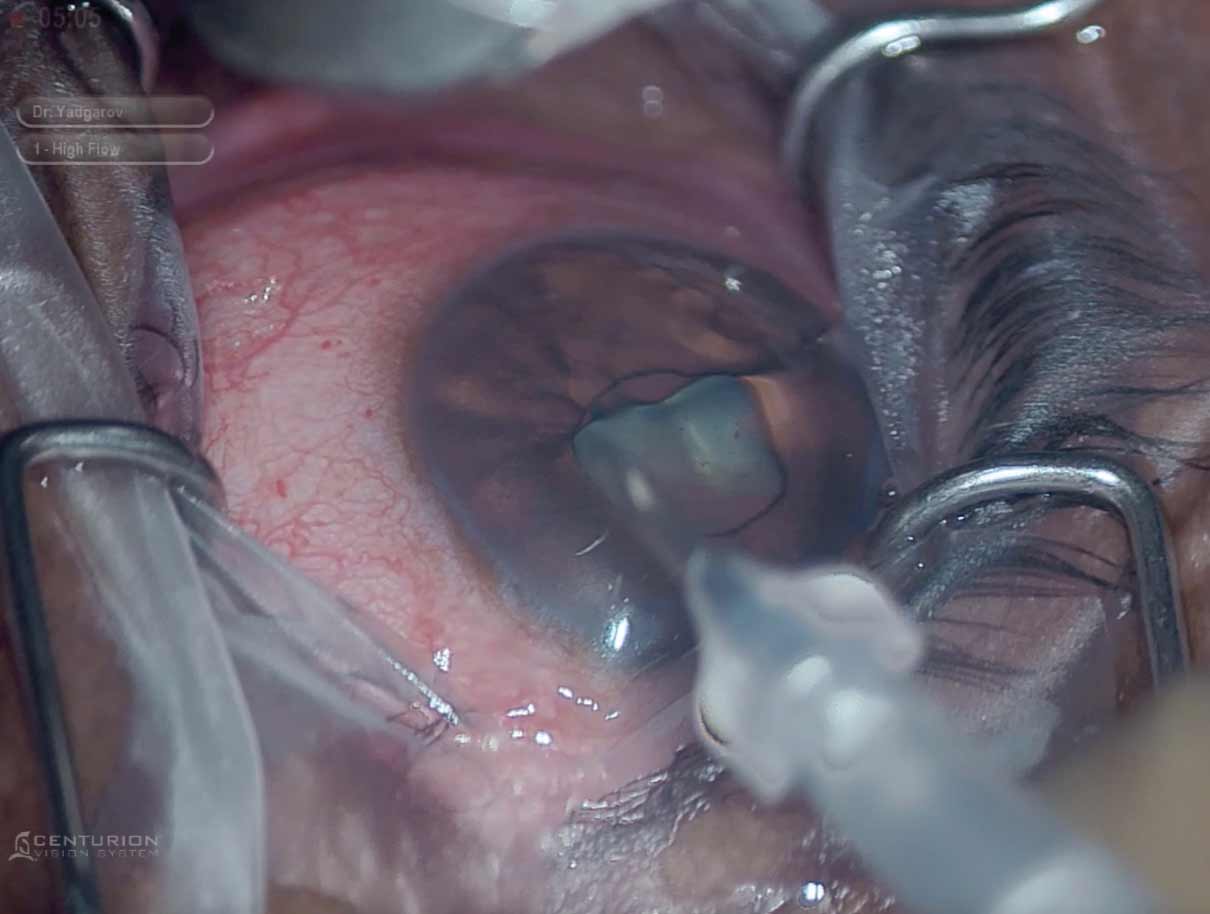 |
| iStent Infinite has proven to be a safe and effective MIGS procedure to lower IOP and improve aqueous outflow. Photo: Arkadiy Yadgarov, MD. Click image to enlarge. |
iDose TR
This implant has been in development for many years and its New Drug Application (NDA) was recently submitted to the FDA. The iDoseTR, also from Glaukos, is an intraocular implant designed to continuously deliver travoprost from within the anterior chamber for extended periods of time. The device is designed such that it can be removed and replaced with a new iDose TR, potentially offering a long-term dropless alternative to a regimen of daily topical therapy.2
The NDA submission includes data from two Phase III pivotal trials, which both successfully achieved the prespecified primary efficacy endpoints through three months and demonstrated tolerability and safety through 12 months. The application also includes data from the iDose TR exchange trial, which included a second administration of the implant and removal of the original iDose TR, with the second administration demonstrating a favorable safety profile over 12 months.2
We have all seen patients who continuously progress no matter our pharmaceutical or surgical treatments and there is a need for effective alternatives, such as stents and drug delivery implants. These new products are a bridge between first-line therapies, potentially a pivotal moment in glaucoma care. Postoperative care is expected to be similar to other low-impact glaucoma procedures; it is important to discuss this with your comanaging surgeon.
1. Sarkisian SR Jr, Grover DS, Gallardo MJ, et al. iStent Infinite Study Group. Effectiveness and safety of iStent Infinite trabecular micro-bypass for uncontrolled glaucoma. J Glaucoma. 2023;32(1):9-18. 2. Glaukos submits new drug application to U.S. FDA for iDose TR. www.investors.glaukos.com/investors/news/news-details/2023/Glaukos-Submits-New-Drug-Application-to-U.S.-FDA-for-iDose-TR/default.aspx. February 27, 2023. Accessed March 20, 2023. |

