 Years ago, most of the general public had never even heard of celiac disease—but today awareness of this increasingly common digestive condition has grown as quickly as the gluten-free sections in local supermarkets. An immune-mediated, chronic inflammatory disorder of the small intestine, celiac disease (CD) is nothing new, but it is definitely more prevalent.
Years ago, most of the general public had never even heard of celiac disease—but today awareness of this increasingly common digestive condition has grown as quickly as the gluten-free sections in local supermarkets. An immune-mediated, chronic inflammatory disorder of the small intestine, celiac disease (CD) is nothing new, but it is definitely more prevalent.
Nearly five times as many Americans have CD now than in the 1950s.1 Estimates suggest that close to 2 million people in the US, or 1% of non-Hispanic Americans, suffer with this debilitating digestive disease. So it’s very likely that you may encounter a patient in your office whose immune system reacts to gluten, a protein found in wheat, barley rye, and to a much lesser extent, oats.
While CD hasn’t been directly correlated with eye disease, there are a number of secondary ocular complications in many of the higher-risk populations. Being aware of a patient’s celiac disease can enable you to be more vigilant and prepared to deal with some of the ancillary complications that may arise.
A Few Basics to Digest
Associated with human leukocyte antigen (HLA) DQ2 and DQ8 haplotypes, CD can occur in individuals with a certain genetic background.2 For these people, eating foods with gluten ultimately causes mucosal damage to the villi lining the intestine, keeping it from properly absorbing nutrients.
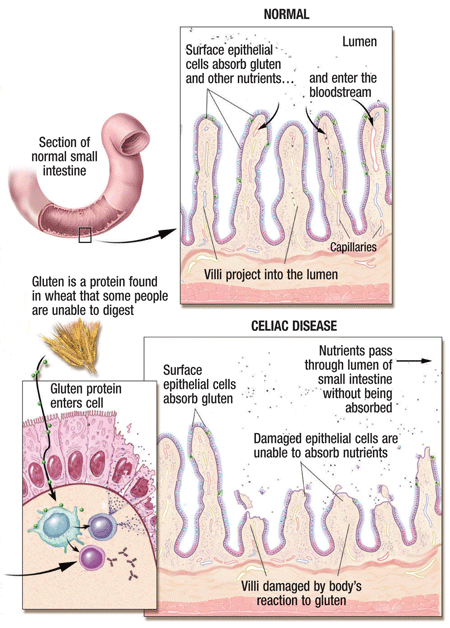
The medical illustration above details the immune reaction that people with celiac disease have when they ingest gluten, damaging the small intestine. Image Courtesy: U.S. Pharmacist
The clinical presentation of CD is age-dependent and quite varied. Classic features in the first few years of life are malnutrition, diarrhea, abdominal pain and distension. Children and adolescents frequently present with short stature and delayed onset of puberty.2,3
Conversely, many patients with CD present at a later age with more subtle symptoms, so the diagnosis may be delayed. They may be symptomatic for years prior to their diagnosis, and are often initially misdiagnosed with irritable bowel syndrome. Adults have diarrhea as a major symptom of CD in approximately 50% of cases.4 Other gastrointestinal symptoms may include abdominal pain, diarrhea or constipation, bloating and excessive gas.
Patients identified with CD by screening due to genetic and other risk factors are often asymptomatic or mildly symptomatic.4 Due to heightened awareness and increased screening efforts, this population is rapidly growing. Some experts believe that something in the environment seems to be triggering the various genetic and biological factors that drive CD.2-4
Diagnostic Work-up for CD
A gastroenterology consult should be obtained when CD is suspected, and/or to screen individuals considered high risk. In addition to a detailed history and physical examination, serologic testing is performed.
There are multiple antibodies found in CD, but endomysial IgA (EMA) and transglutaminase (TG) IgA autoantibodies are the most sensitive and specific.2 If serologic testing is positive, intestinal biopsy via endoscopy of the small intestine is required for confirmation.2,4,5
IgA deficiency is increased in people with CD. If the patient is IgA deficient, tissue transglutaminase IgG can be measured.
When CD is suspected clinically in the presence of IgA deficiency, upper intestinal endoscopy with biopsy should be considered, regardless of the autoantibody results.4
Associated Conditions and Secondary Complications
Patients with celiac disease have an increased risk of certain malignancies, the most feared complication of CD.3 Adenocarcinoma of the small intestine is rare, though the risk for this carcinoma is increased in people with CD. When CD is not recognized and/or treated, complications commonly develop, including iron and other nutritional deficiencies, osteopenic bone disease and growth retardation in children. There are also issues related to fertility: increased rates of infertility and spontaneous abortions.2,3
In these populations, many of which have significant ocular complications, the rates of celiac disease range from 5%-10%.
Several conditions are found with an increased frequency in CD, though not thought to be due to gluten ingestion. The known association between CD and type 1 diabetes is likely related to a shared genetic risk.5 Autoimmune thyroid disease also shares genetic risk factors with CD. In addition, CD has been found at an increased rate in patients with both Turner and Down syndromes.2,3,6
Populations at Increased Risk for CD2-6
(both hyper- and hypothyroidism)
Direct ocular complications of CD have not been identified; however, ocular complications may occur secondary to associated conditions, such as type 1 diabetes (early cataract as in our case report, as well as diabetic retinopathy and diabetic macular edema) and anemia (optic neuropathy).
If not properly diagnosed or treated, celiac disease may cause a patient’s diabetic retinopathy to progress. Malabsorption of vitamins A and E may result in a pigmentary retinal degeneration not unlike retinitis pigmentosa.
Treatment Approaches
Lifelong avoidance of gluten is the primary treatment for CD. Consultation with a dietician for strict gluten-free diet education is essential in both treatment and follow-up. Certain grains, such as oats, can be contaminated with wheat during growing and processing, so dietitians generally recommend avoiding oats unless they are specifically labeled gluten-free.2,3
Lactose intolerance may be a side effect of CD, since the damaged small intestine cannot break down the lactose molecule. Patients should avoid dairy products until the intestinal symptoms have improved, and use dietary substances such as folate, iron, calcium and vitamins in the early stage of the disease as well.2
Serologic testing is used to monitor therapy, as antibody levels are expected to decline with treatment. A small subset of treated patients may fail to respond to a gluten-free diet. In some, corticosteroids might be helpful (in which case, you will want to monitor the patient’s intraocular pressure).6 In those that fail to respond to steroids, other comorbidities, such as abnormalities of the small intestine, have to be ruled out.
Early serologic diagnosis and dietary treatment in celiac disease can prevent severe, sometimes life-threatening, complications.
1. Rubio-Tapia A, Kyle RA, Kaplan EL, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology. 2009 Jul;137(1):88-93.
2. Papadakis M, McPhee S. GI Disorders. In: Current Medical Diagnosis Treatment 2013. 52nd ed. Chicago: McGraw Hill Lange;2009:621-3.
3. Crowe SE. Celiac Disease. In: DeLegge MH (ed.). Clinical Gastroenterology: Nutrition and Gastrointestinal Disease. Totowa, NJ: Humana Press Inc.;2008:123-47.
4. Green PH, Cellier C. Celiac disease. N Engl J Med. 2007 Oct 25;357(17):1731-43.
5. Barker JM, Yu J, Yu L, et al. Autoantibody “sub-specificity” in type 1 diabetes: risk for organ-specific autoimmunity clusters in distinct groups. Diabetes Care. 2005 Apr;28(4):850-5.
6. Turner SM, Moorghen M, Probert CS. Refractory coeliac disease: remission with infliximab and immunomodulators. Eur J Gastroenterol Hepatol. 2005 Jun;17(6):667-9.
Her
systemic history was remarkable for type 1 diabetes of 28 years
duration, which was being treated with insulin. She reported improved
glycemic control following the start of a gluten-free diet. • Diagnostic data. Refraction
improved her best-corrected visual acuities from 20/40 OD and OS to
20/25 OD and OS. BCVA was 20/25 due to cortical cataract on the visual
axis in each eye. Clinical examination showed mild cortical lens
opacities. Dilated funduscopy ruled out signs of diabetic retinopathy. • Management.
A new spectacle prescription with anti-reflective lenses was issued.
The patient was educated about the relationship between celiac disease
and type 1 diabetes. She was advised to continue with her primary
physician’s dietary recommendations, as better glycemic control may help
her avoid ocular and other complications.
Case Report
• History. A 40-year-old
white female presented complaining of gradual, bilateral blur and
increased glare while driving at night. The patient was only recently
diagnosed with celiac disease, although she had suffered intermittent
bouts of diarrhea, bloating, upset stomach and weight loss for most of
her adult life.

