It’s no secret that Americans are in the midst of a substance abuse crisis. According to the NIH, more than 33,000 Americans died from opioid overdoses in 2015 alone.1 That same year, approximately two million Americans suffered from substance abuse disorders related to prescription opioid pain relievers, 591,000 from heroin use alone.2 The cost can be devastating, but substance abuse is a modifiable lifestyle factor. As primary care physicians, optometrists can play a role in recognizing damage or dysfunction to either ocular structures or the components of the visual pathway these drugs cause and counseling patients in these circumstances.
This article reviews commonly used legal and illicit substances, and how each are associated with the formation, or exaggeration, of disease or damage.
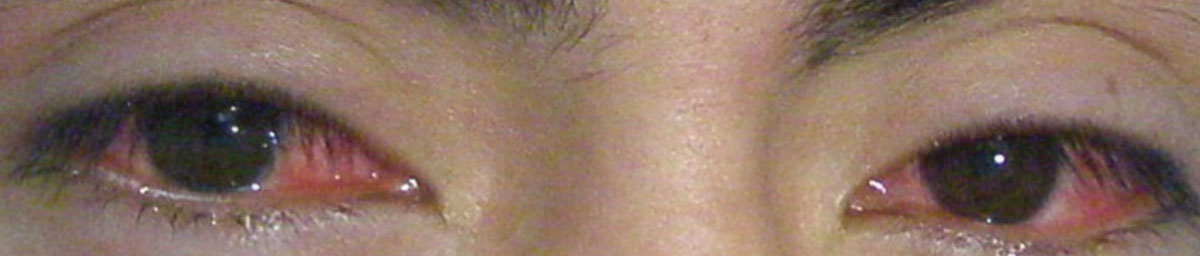 |
| This patient displays toxic conjunctivitis secondary to methamphetamine use. |
Caffeine
The average cup of coffee or tea (in the United States) contains between 40mg and 150mg of caffeine.3,4 Over-the-counter commercially available caffeine supplements contain between 100mg and 200mg per unit.3,4 It is not until an individual ingests in excess of 5g of caffeine that toxicity is observed.3
Lid and cornea impact. Excess caffeine is associated with eyelid myokymia.5,6 In animal models, during prenatal development ingested caffeine caused decreased total corneal thickness; it changed the thickness of each corneal layer in chicken embryos via changes in structure and the amount of collagen fibers.7,8
Caffeine consumption increases pupil size and amplitude of accommodation and can even dampen spontaneous pupillary oscillations up to six and a half hours after ingestion.9
Glaucoma. Although previous reports indicated that coffee consumption (and by extension, caffeine) raised intraocular pressure (IOP), more recent studies could not elicit a statistically significant change.10-16 The rise in previous studies was likely secondary to water absorption.15,16 In fact, more recent research lauds the potential use of caffeine to decrease ocular hypertension and attenuate neuroinflammatory responses, particularly in reducing the loss of retinal ganglion cells in ocular hypertensives.17,18
Posterior segment. Additionally, caffeine consumption is associated with decreased choroidal thickness at least four hours after ingestion.19 Highly caffeinated energy drink consumption can cause intraretinal hemorrhages and acute loss of vision, which may be irreversible.20 Similarly, excessive energy drink consumption can lead to transient macular ischemia via choroidal vasoconstriction, which causes bilateral central scotomas.21 With the increased frequency of these types of occurrences, these retinal findings have been termed “coffee and donut maculopathy” or “energy drinkers’ maculopathy.”22 With these ocular effects in mind, it is recommended that daily energy drink dosage should not exceed 400mg/day.23
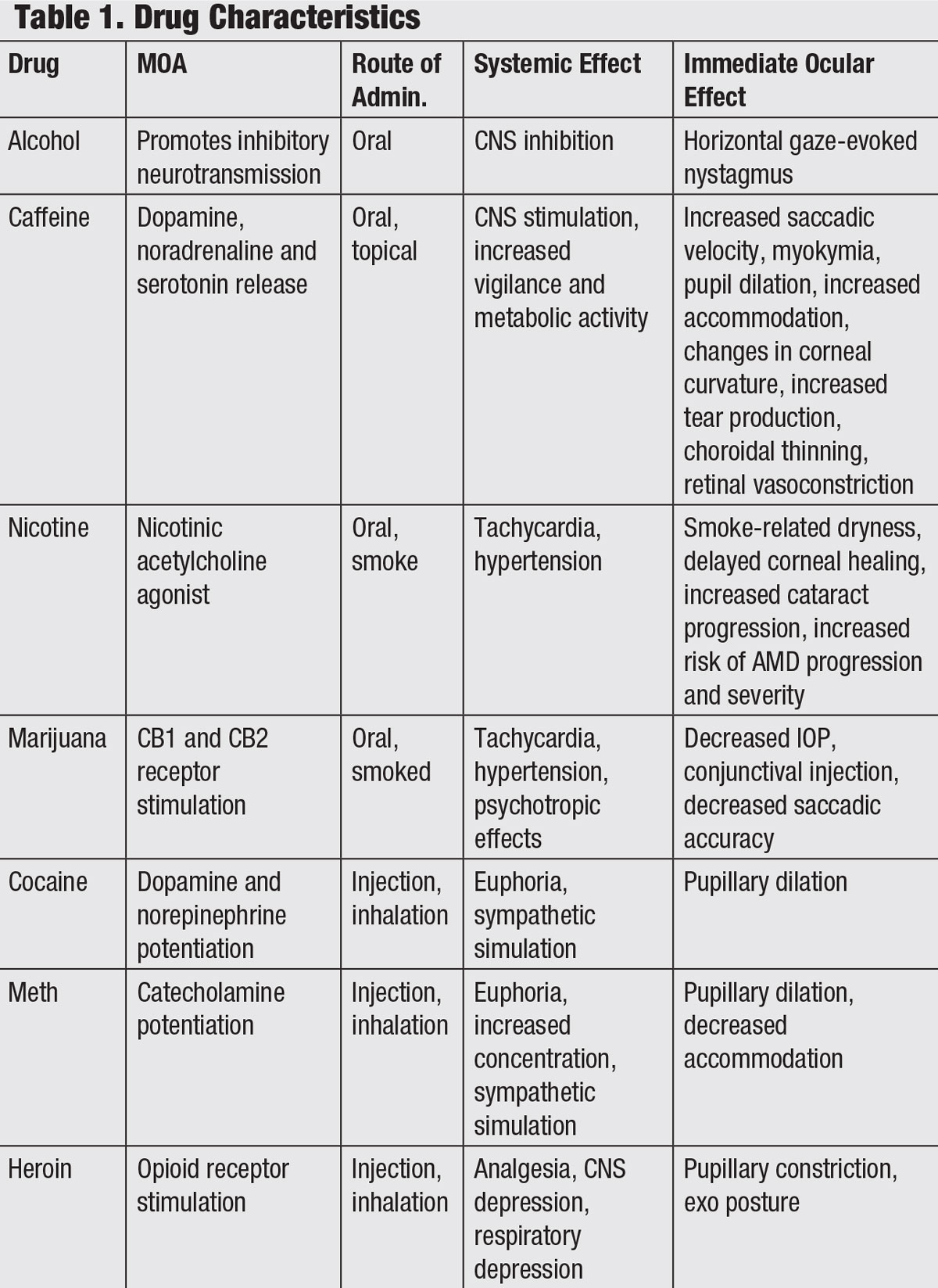 |
| Click table to enlarge. |
Alcohol
The most commonly abused substance in the United States, alcohol accounts for 3.5% of deaths annually.24,25 It should go without saying that excess consumption creates adverse effects which include liver cirrhosis, neurotoxicity and carcinogenesis.25
Macular degeneration. When it comes to ischemic heart disease, alcohol consumption has a J-shaped curve, meaning the dose-risk association shows a clear benefit in moderate drinkers but an increased risk as consumption increases to abusive levels.25 That J-shaped curve applies to age-related macular degeneration (AMD) as well.25 Some studies show a protective effect against AMD in moderate wine drinkers but a 20% increase in the development of AMD when alcohol consumption exceeds three drinks per day.25,26 The pathophysiology of this double-edged effect is uncertain, but researchers theorize that the benefit of moderate drinking comes from raised levels of high-density lipoproteins.26
Cataract development has been linked to alcohol abuse through a proposed mechanism of metabolic byproducts such as acetoaldehyde which reacts with and modifies lens proteins causing opacification of the crystalline lens.26
Ocular motility disturbance. Alcohol also contains ethanol, a central nervous system depressant, which exerts its effects first on higher-order functions, such as reasoning, judgement and memory, and then on lower-order functions such as speech, gait and balance. As blood alcohol concentration (BAC) increases, multiple cortical structures are implicated including those that control voluntary eye movements.27-44 Alcohol can decrease maximum saccade velocity by 17% to 19%.28 Smooth pursuits are affected by increased saccadic intrusions. Doll’s head eye movements, a reflex originating in the brainstem, remain unaffected.26,28 One study shows an alcohol-induced effect on ocular alignment via changes in distance and near phoric deviations.28 Patients with alcohol abuse issues often enter into presbyopia prematurely.29
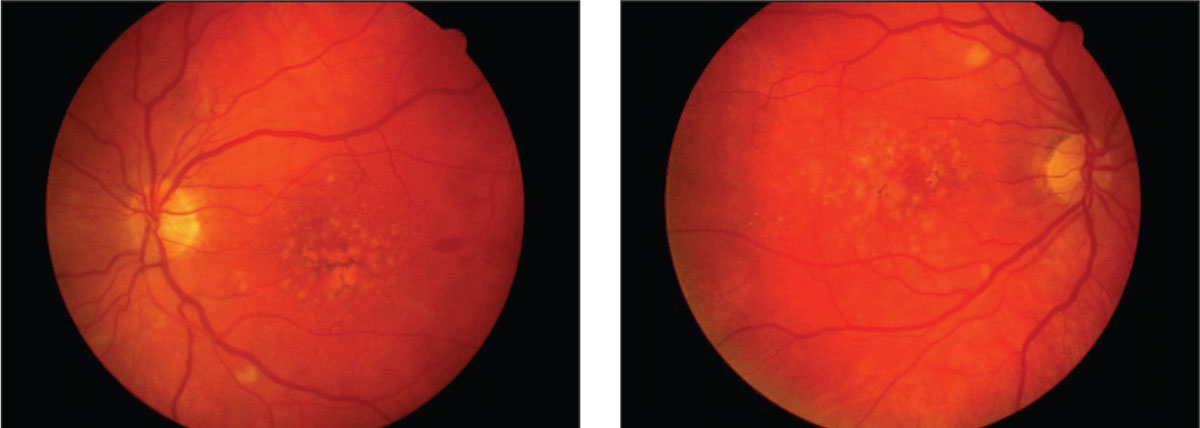 |
| Macular degeneration, as demonstrated in these fundus images, is associated with the abuse of a number of illicit substances, including alcohol. Click image to enlarge. |
Optic nerve and neurological impact. ‘Tobacco-alcohol amblyopia’ is characterized by a central or paracentral scotoma, color vision defects and optic nerve pallor in heavy drinkers and smokers.30 Originally presumed to be caused only by tobacco and alcohol toxicity, it is now known that the dominant pathologic factor is vitamin deficiencies brought about by the nutritional neglect seen in heavy drinkers.30 Wernicke’s encephalopathy, a neurological syndrome caused by alcohol abuse, can cause horizontal nystagmus, gaze nystagmus, and disc edema.31-33 Fetal alcohol syndrome affects ocular tissues the most and can cause optic nerve hypoplasia, retinal vessel tortuosity, strabismus, saccadic dysfunction, coloboma, microphthalmia and corneal clouding.26,34,35
Tobacco
Cigarette smoke’s inflammatory effects can impact both the eye’s vasculature and the ocular surface.
Ocular surface and anterior segment. Cigarette smoking increases the risk of dry eye disease (DED), due to the smoke acting as a direct irritant to the eyes.36,37 In patients already suffering from DED, smoking increases burning and foreign body sensation.37,38 Smoking lowers tear break-up time, lowers mean Schirmer scores, decreases corneal and conjunctival sensitivity and increases punctate staining.39,40 Decreased corneal and conjunctival sensitivity is associated with significant loss of goblet cells, squamous metaplasia and altered tear proteins in smokers.37,41
One mechanism responsible for dry eye symptoms is lipid peroxidation of the outer layer of the tear film, leading to tear film instability.40 Specifically, lipid peroxidation leads to nonuniform spreading of tears and patchy lipid layer thickening; this, particularly of the nonpolar oils, alters the function of the mucin layer, which makes the corneal surface unwettable.37,42
Cigarette smoking is also associated with delayed corneal epithelial healing, persistent clinical and subclinical corneal abrasions and chronic keratitis and progression of Fuchs’ endothelial corneal dystrophy.39,43,44 Similarly, current smokers have an increased risk of delayed treatment response in episcleritis and scleritis compared to nonsmokers.45 The nicotine found in tobacco products is associated with decreased central corneal thickness via increased oxidative burden leading to endothelial cell apoptosis and reduced endothelial function.39,43
Cataracts. Smoking is a strong risk factor for the hastened development of age-related nuclear cataracts in a dose-dependent fashion.46-49 The pathogenesis of cataract formation is hastened via direct oxidative damage, direct toxicity to the lens and reduced antioxidant levels.39
Ocular and systemic issues. Smoking during pregnancy increases risk of poor stereoacuity via interruption of central fusion development in the fetus. It also increases the risk of strabismus; specifically, congenital esotropia and exotropia.50,51 Smoking exacerbates Graves’ hyperthyroidism and thyroid orbitopathy.52,53
 |
| These eyes display branch retinal artery occlusion. Tobacco use in particular can contribute to problems in the eye’s vasculature system. Click image to enlarge. |
Glaucoma. Smoking is associated with increased IOP and reduced choroidal blood flow, leading to increased resistance to aqueous humor leaving the anterior chamber.54,55 Smoking is associated with increased intraocular pressure and increases the risk of primary open angle glaucoma (POAG) compared with former or non-smokers; heavy smokers have significantly higher risk.56,57 Additionally, the course and progression of POAG appears to be hastened by smoking.57 This is due to an increased inflammation and apoptosis marker levels in the aqueous humor and plasma. While some evidence supports increased outflow facility secondary to nitric oxide derived from vascular endothelial cell processes, this is offset by decreased trabecular meshwork cell volume.58-60
Retina. Smoking impedes blood flow directly to the macula and increases inflammation, promoting macular degeneration.61,62 Cigarette smoking coupled with a genetic susceptibility increases the risk of AMD synergistically.63 It can also increase the risk of first-time uveitis, bilateral uveitis, panuveitis and idiopathic cystoid macular edema (CME) as well as CME after cataract surgery.64 The smoking of any substance confers a threefold risk of retinal vein occlusion (RVO) and creates circumstances that provoke or accelerate cardiovascular disease risk.23 Any patient with an occlusion or RVO should be educated on the cessation of smoking (anything) to prevent comorbidities and further vascular damage.42
Marijuana
A paucity of evidence exists for the direct ocular side effects of cannabis. Marijuana increases sympathetic nervous system activity, increasing heart rate and blood pressure via Delta 9-tetrahydrocannabinol (Δ9-THC).65 Cannabinoid use is associated with similar ocular signs, including conjunctival hyperemia, chemosis, severe corneal opacification and neurotoxicity.66
Glaucoma. Although cannabinoids are effective in reducing IOP, their therapeutic use is precluded due to short duration of action, receptor desensitization and association with behavioral side effects.67 There is ongoing research in isolating key active compounds and endocannabinoid receptors in an effort to create an effective therapeutic strategy, while avoiding retinal ganglion cell dysfunction and the functional side effects.66,68
Visual performance. Cannabis has a direct effect on short term memory and eye movements such as decreased saccadic accuracy, and decreased smooth pursuit eye movements, leading to trouble reading, trouble tracking, decreased visual search capabilities, decreased ability to detect peripheral stimuli.69 Cannabis use also leads to color discrimination distortions, changes in pupil size, reduced accommodative range, decreased acuity and increased photophobia.70,71 Chronic cannabis use is associated with distortions in depth perception, color discrimination, pattern recognition and visual perception.72 Evidence exists both for and against night vision with improvement with cannabinoids.70,73,74 Although some studies find decreased dark adaptation abilities, others have found a decreased a-wave amplitude of the full-field electroretinogram in scotopic conditions after acute cannabis inhalation.70,73,74
Cocaine
This drug’s primary mechanism of action is inhibition of dopamine reuptake, making it a powerful agent of long term addiction.75-77 With respect to the eye, the immediate effects of cocaine include mydriasis, lid retraction, conjunctival blanching and decreased corneal sensation.78,79
Cornea. Cocaine abuse (whether via smoking or snorting) may lead to a condition called crack cornea, a well-reported syndrome of chronic corneal toxicity ranging in severity from mild punctate keratitis to severe bilateral infectious ulcers.80,81 Although the mechanism is unknown, it has several known contributors. Snorted cocaine, absorbed through the nasal mucosa, produces bilateral keratitis (worse on the side most frequently used for snorting) secondary to corneal nerve devitalization.75 Smoked cocaine makes direct contact with the cornea and acts like most topical anesthetics, softening the cornea and indirectly reducing the blink reflex.80,81 Aerosolized adulterants such as talc, sugar, flour, starch or procaine may cause surface damage as well.82
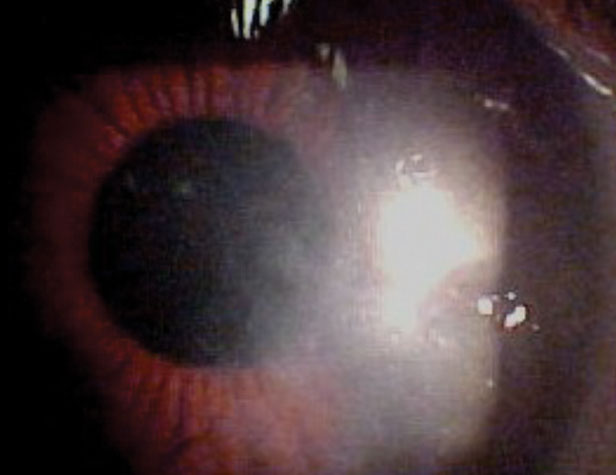 |
| Perhaps due to its method of delivery, snorting, cocaine abuse can lead to corneal ulcers, as seen here. |
From direct irritation of the ocular surfaces to exposure keratopathy, mechanical damage from rubbing, secondary infectious keratopathy with common or atypical organisms (Streptococcus mitis, Capnocytophaga and Candida albicans), crack smoke has the potential to create vision-threatening keratopathy.80 Management is often made difficult due to problems with compliance and follow up.83 Hospital admission may be helpful to prevent re-use in the acute infection period.80
Orbital inflammation. Nasally inhaled cocaine is locally destructive to the nasal mucosa and supporting bony tissues due to vasoconstrictive ischemia and toxic contaminants.84 This may lead to bony-destructive focal inflammatory granulomatous lesions as well as recurrent infections, which cause further erosion of the paranasal sinuses orbital tissues.84
Inflammatory effects may also create orbital complications on the affected side such as extraocular muscle inflammation, nasolacrimal duct obstruction, orbital apex syndrome, orbital cellulitis, optic neuropathy, optic perineuritis and central retinal vein occlusion (CRVO).85,86 Vision loss from optic neuropathy may occur via compressive, infiltrative or ischemic mechanisms.86 Neuroimaging shows characteristic bony destruction of the nasal and paranasal sinuses and nasal septum.86 Opportunistic infections, such as mucormycosis or bacterial orbital cellulitis, may be visually devastating and life-threatening.87
Retinal vasculature. Ocular vascular sequelae from any mode of intake may result from chronic cocaine use secondary to its sympathetic effects created by increased norepinephrine and resulting vasospasm, hypertension and atherosclerosis. Reported retinal vascular complications include central retinal artery occlusion, cilioretinal artery occlusion, intraretinal bleeding and CRVO.75 Additionally, cutting agents such as talcum powder (magnesium silicate) can deposit in retinal arterioles after chronic intravenous administration producing embolic sequellae.75,78 These particles appear as fine, refractile yellow-white crystals in the inner retinal layers and have been found in patients who inject other illicit substances such as heroin and methylphenidate.88,89
Retinal complications include ischemic atrophy of the inner retinal layers as well as the formation of peripheral retinal neovascularization (sea fans).88 Depending on the location of obstruction, this may result in vision loss, vitreous hemorrhage and tractional retinal detachment.88 Duration and severity of drug use seems to be the dominant factor in the severity of presentation and complications that arise.88
Heroin
Opiates have been used for effective pain relief for millennia.90 However, their proclivity for dependence has led to widespread abuse of both prescription and illicit opiate forms.91
Retina. As with other intravenously administered illicit drugs, talc retinopathy can develop, appearing as small white refractile particles visible on funduscopy, leading to focal retinal ischemia and peripheral retinal neovascularization.34 Similar to cocaine, heroin—when inhaled nasally—can result in inflammation and infection, including fungal mucormycosis.92 Other ocular adverse effects of injected heroin are endogenous infectious endophthalmitis and toxoplasmic chorioretinitis.92-98
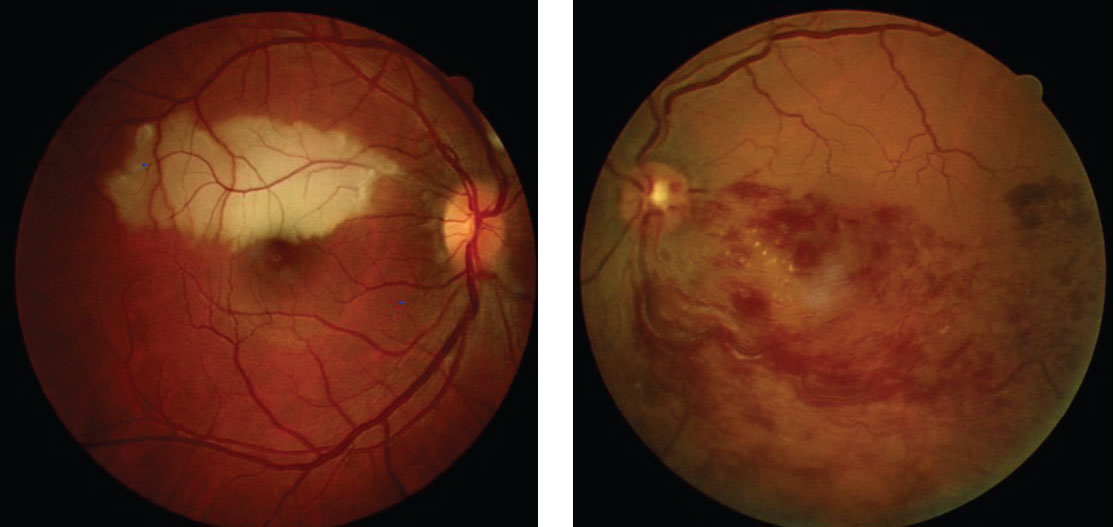
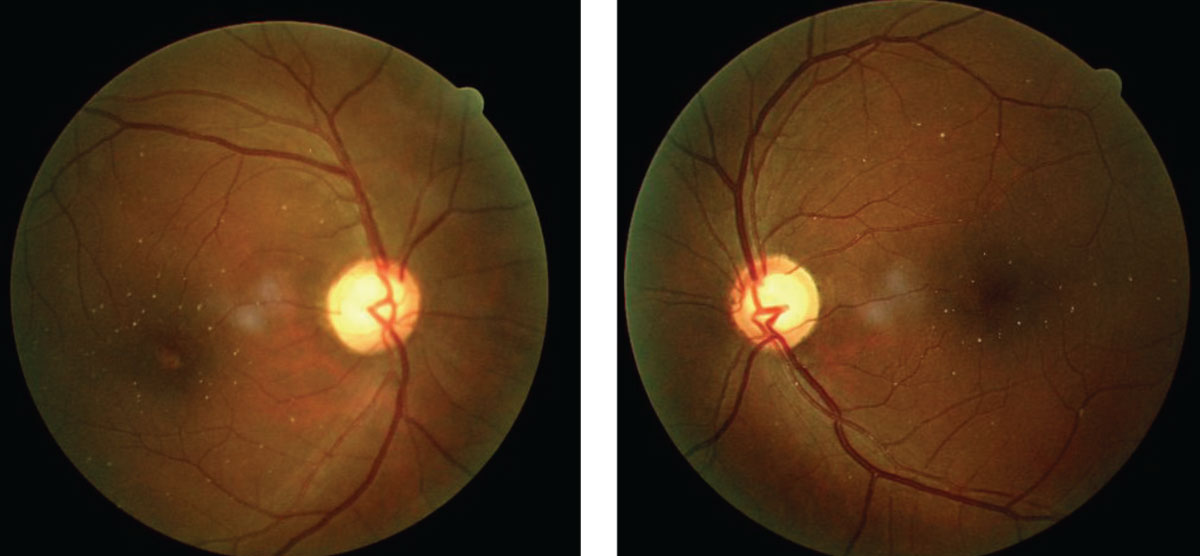 |
| Talc retinopathy, as shown in these fundus images, is associated with heroin use. Click image to enlarge. |
Binocular system. While exo posture and pupillary miosis are associated with opioid dependence, opioid withdrawal is associated with eso posture and pupillary mydriasis.99-101 A comitant acute eso deviation may develop and last for months after detoxification.99,100 This finding does not involve cranial nerve VI pathology and is not correlated with a hyperopic shift.99,100 There is no accepted mechanism for this; however, some theories assert that overactive accommodative convergence is the driving force (spasm of the near reflex).99 This seems to suggest that both opioid use and withdrawal creates a disequilibrium within the accommodative triad of miosis, convergence and accommodation, likely due to the disruption of normal mu opioid receptor activity in the midbrain.99,100
Fetal development. Neonatal abstinence syndrome is a multisystem disorder in infants who experience opioid withdrawal from maternal opioid use. It has strong associations with certain ophthalmic abnormalities including pendular horizontal nystagmus, abnormal visual evoked potential, delayed visual maturation and strabismus.102-105
Methamphetamines
Methamphetamine is a strong central nervous system stimulant.106-109 It increases the amount of dopamine and other catecholamines released by preventing their breakdown and reuptake.107,110 Direct sympathetic stimulation induced by methamphetamine causes acute pupillary dilation as well as blurred vision secondary to decreased accommodation.107
Acute vascular complications may occur with methamphetamine use due to acute blood pressure elevation related to vasospasm and an increased heart rate.107,110 This may manifest as hemorrhagic stroke, intracranial hemorrhage, intra-retinal hemorrhage or a non-ischemic optic neuropathy–type presentation.106,107,110,111 Methamphetamine use is also associated with episcleritis, scleritis and retinal vasculitis resembling that seen in polyarteritis nodosa.106
Cornea. Ulceration is prevalent among methamphetamine users due to a variety of possible mechanisms, including the pharmacological effects of the drug, the effects of cutting agents and effects related to the route of administration.106,110 Corneal nerves have a high concentration of dopamine receptors and may be target of neurotoxicity mediated by excessive dopamine production.107 Damage to corneal sensory nerves can result in a neurotrophic keratitis and corneal ulceration.112
Elevation of the pain threshold during methamphetamine use may decrease the blink reflex and predispose to exposure keratopathy.110 Diluent additives such as lidocaine may further weaken the epithelium and lead to ulceration.110 Nasal inhalation brings methamphetamine into both spatial and circulatory proximity to the eye and may increase the risk of keratitis.110 Compounding the issue, mental effects of the drug such as increased awareness, heightened concentration and irritability result in excessive and harmful rubbing and scratching of the eyelids and ocular surface if symptoms develop.110
Alcohol Threatened My Life,Optometry Saved Me—Message from an optometrist in recovery I’m John B. and I’m an alcoholic. I grew up in what I thought was a normal household. I was first exposed to alcohol as a teenager, when I realized my father was an alcoholic, and started drinking myself around 15 years old. When I finally graduated high school (just barely), I enlisted in the Army and did a few tours overseas. I trained to be an airplane mechanic, but in my free time I would binge drink. I returned home a good mechanic and a better drinker. That’s when I fell into a deep depression and soothed with at least one bottle daily. This went on for a long time and I started noticing trouble with my vision. So, I set up an appointment with an optometrist. He asked me about my health and if I smoke, take drugs or use alcohol. I said no. From there he proceeded with rest of the exam and told me I was a little nearsighted but otherwise OK. He seemed like someone I could talk to, and I finally admitted I did drink “once in a while” and asked if that caused my nearsightedness. He said no, but he asked how much I drink a day. I told him a couple of beers (the standard answer) and he laughed and in a good-natured way. The jig was up. My exam was over, but the doc said, “Let’s talk about this for few more minutes.” He revealed that he had an alcoholic relative who struggled many years, to the point where he had developed serious medical problems. He was ashamed of his alcoholism, much like I was, and was reluctant to seek help. He finally did, though. “It’s never too late,” the doctor told me. This interaction turned out to be one of the most important moments of my life. He directed me to a 12-step program that required me to faithfully attend meetings with other alcoholics. I’ve been sober now for three years. I have a great job, attend meetings regularly and even reach out to other alcoholics. I know it sounds funny, but an eye doctor actually saved my life! I always wondered if he had special training in optometry school, but all it really took was a little compassion, asking simple questions and being prepared to listen and respond. |
Systemic Impact
When an optometrist uncovers a substance abuse disorder with a direct bearing on the patient’s ocular and systemic health, the opportunity arises to provide initial counseling and an appropriate referral to a primary care physician or counseling center. Familiarity with the existing local network will be helpful here but the patient’s primary care physician or the local emergency room may be the most appropriate referrals depending on the severity of the situation.
Initial counseling can be performed in the exam room and should start with the basics: the patient has a vision-threatening condition with modifiable lifestyle factors. Explaining the connection between their behavior and their vision may strike a chord in the patient’s mind and be the catalyst to changing their behavior.
The American Society of Addiction Medicine has nationally established criteria for placement and treatment of patients with addiction and co-occurring conditions. Emergency room and primary care physicians will be familiar with the particulars of these guidelines and may be the most appropriate referral sites depending on the urgency of the situation.113 Depending on the severity of their substance abuse disorder, patients can expect an individually tailored treatment plan on the continuum ranging from intensive medical care to outpatient services.
Dr. Karbach is a clinical instructor at The Eye Institute, Pennsylvania College of Optometry at Salus University.
Dr. Kobrenko practices at Bucks-Mont Eye Associates and Visionworks.
Dr. Myers is senior staff optometrist at the Coatesville Veterans Affairs Hospital in Pennsylvania.
Dr. Gurwood is a professor at Salus University.
|
1. Rudd R, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. CDC Morbidity and Mortality Weekly Report. 2016;65(50-51):1445–52. 2. Center for Behavioral Health Statistics and Quality. (2016). Key substance use and mental health indicators in the United States: Results from the 2015 National Survey on Drug Use and Health (HHS Publication No. SMA 16-4984, NSDUH Series H-51). Retrieved from www.samhsa.gov/data/. 3. Kerrigan S, Lindsey T. Fatal caffeine overdose: two case reports. Forensic Sci Int. 2005;153(1):67-9. 4. Stohs S, Badmaev V. A review of natural stimulant and non-stimulant thermogenic agents. Phytother Res. 2016;30(5):732-40. 5. Eyelid Disorders. National Eye Institute. www.nei.nih.gov/faqs/eyelid-disorders-myokymia-twitching-eye. Accessed December 11, 2017. 6. Banik R, Miller N. Chronic myokymia limited to the eyelid is a benign condition. J Neuroophthalmol. 2004;24(4):290-2. 7. Kujawa-Hadrys M, Tosik D, Bartel H. Changes in thickness of each layer of developing chicken cornea after administration of caffeine. Folia Histochem Cytobiol. 2010;48(2):273-7. 8. Monika K, Dariusz T, Hieronim B. Ultrastructural changes in the developing chicken cornea following caffeine administration. Folia Histochem Cytobiol. 2010;48(3):371-6. 9. Abokyi S, Owusu-Mensah, Osei KA. Caffeine intake is associated with pupil dilation and enhanced accommodation. Eye (Lond). 2017;31(4):615-9. 10. Avisar R, Avisar E, Weinberger D. Effect of coffee consumption on intraocular pressure. Ann Pharmacother. 2002;36(6):992-5. 11. Pasquale LR, Kang JH. Lifestyle, nutrition, and glaucoma. J Glaucoma. 2009;18(6):423-8. 12. Li M, Wang M, Guo W, et al. The effect of caffeine on intraocular pressure: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2011 Mar;249(3):435-42. 13. Kang JH, Willett WC, Rosner BA, et al. Caffeine consumption and the risk of primary open-angle glaucoma: a prospective cohort study. Invest Ophthalmol Vis Sci. 2008;49(5):1924–31. 14. Chandrasekaran S, Rochtchina E, Mitchell P. Effects of caffeine on intraocular pressure: the Blue Mountains Eye Study. J Glaucoma. 2005;14(6):504-7. 15. Chandra P, Gaur A, Varma S. Effect of caffeine on the intraocular pressure in patients with primary open angle glaucoma. Clin Ophthalmol. 2011;5:1623-9. 16. Dervisogulları MS, Totan Y, Yüce A, et al. Acute effects of caffeine on choroidal thickness and ocular pulse amplitude. Cutan Ocul Toxicol. 2016;35(4):281-6. 17. Madeira MH, Ambrósio AF, Santiago AR, et al. Caffeine administration prevents retinal neuroinflammation and loss of retinal ganglion cells in an animal model of glaucoma. Sci Rep. 2016;6:27532. 18. Boia R, Ambrósio AF, Santiago AR. Therapeutic Opportunities for Caffeine and A2A Receptor Antagonists in Retinal Diseases. Ophthalmic Res. 2016;55(4):212-8. 19. Zengin MO, Cinar E, Karahan E, et al. The effect of caffeine on choroidal thickness in young healthy subjects. Cutan Ocul Toxicol. 2015;34(2):112-6. 20. Asensio-Sánchez VM. Energy drinks and visual health. Arch Soc Esp Oftalmol. 2014;89(11):467. 21. Lotfi K, Grunwald JE. The effect of caffeine on the human macular circulation. Invest Ophthalmol Vis Sci. 1991;32(12):3028-32. 22. Kerrison J, Pollock S, Biousse V, et al. Coffee and doughnut maculopathy: a cause of acute central ring scotomas. Br J Ophthalmol. 2000;84(2):158-64. 23. Kolar P. Risk Factors for Central and Branch Retinal Vein Occlusion: A Meta-Analysis of Published Clinical Data. J Ophthalmol. 2014(6):724780. 24. Perloff BP, Rizek RL, Haytowitz DB, Reid PR. Dietary intake methodology. II. USDA’s Nutrient Data Base for Nationwide Dietary Intake Surveys. J Nutr. 1990;120(11) :1530-4. 25. Madeleine KMA, Chong EW, Williamson E, et al. 20/20—Alcohol and Age-related Macular Degeneration: The Melbourne Collaborative Cohort Study. Am J Epidemiol 2012;176(4):289-98. 26. Hiratsuka Y, Li G. Alcohol and eye diseases: a review of epidemiologic studies. J Stud Alcohol. 2001;62(3):397-402. 27. Xu L, You QS, Jonas JB. Prevalence of alcohol consumption and risk of ocular diseases in a general population: The Beijing Eye Study. Ophthalmology 2009;116(10):1872-9. 28. Wilkinson IM. The influence of drugs and alcohol upon human eye movement. Proceedings of the Royal Society of Medicine. 1976;69(7):479-80. 29. Campbell H, Doughty MJ, Heron G, Ackerley RG. Influence of chronic alcohol abuse and ensuing forced abstinence on static subjective accommodation function in humans. Ophthalmic Physiol Opt. 2001;21(3):197-205. 30. Grzybowski A, Holder GE. Tobacco optic neuropathy (TON) - the historical and present concept of the disease. Acta Ophthalmol. 2011;89(5):495-9. 31. Day GS, del Campo CM. Wernicke encephalopathy: a medical emergency. CMAJ : Canadian Medical Association Journal. 2014;186(8):E295. 32. Manzo G, De Gennaro A, Cozzolino A, et al. MR Imaging Findings in Alcoholic and Nonalcoholic Acute Wernicke’s Encephalopathy: A Review. BioMed Research International. 2014:503596. 33. Thomson AD, Marshall EJ. The natural history and pathophysiology of Wernicke’s encephalopathy and Korsakoff’s psychosis. Alcohol and Alcoholism. 2005;41(2):151-8. 34. Peragallo J, Biousse V, Newman NJ. Ocular manifestations of drug and alcohol abuse. Current opinion in ophthalmology. 2013;24(6):566-73. 35. Brennan D, Giles S. Ocular involvement in fetal alcohol spectrum disorder: a review. Curr Pharm Des. 2014;20(34):5377-87. 36. Masmali AM, Al-Shehri A, Alanazi SA, et al. Assessment of Tear Film Quality among Smokers Using Tear Ferning Patterns. J Ophthalmol. 2016(11):8154315. 37. Altinors DD, Akça S, Akova YA,et al. Smoking associated with damage to the lipid layer of the ocular surface. Am J Ophthalmol. 2006;141(6):1016-21. 38. Galor A, Lee DJ. Effects of smoking on ocular health. Curr Opin Ophthalmol. 2011;22(6)(11):477-82. 39. Nita M, Grzybowski A. Smoking and eye pathologies. a systemic review. Part I. Anterior eye segment pathologies. Curr Pharm Des. 2017;23(4):629-38. 40. Cooke JP. New insights into tobacco-induced vascular disease: clinical ramifications. Methodist DeBakey Cardiovascular Journal. 2015;11(3):156-9. 41. Matsumoto Y, Dogru M, Goto E, et al. Alterations of the tear film and ocular surface health in chronic smokers. Eye. 2008;(22):961–8. 42. Xu L, Zhang W, Zhu XY, et al. Smoking and the risk of dry eye: a Meta-analysis. Int J Ophthalmol. 2016;9(10):1480-6. 43. Jetton JA, Ding K, Kim Y, et al. Effects of tobacco smoking on human corneal wound healing. Cornea. 2014;33(5):453-6. 44. Zoega GM, Fujisawa A, Sasaki H, et al. Prevalence and risk factors for cornea guttata in the Reykjavik Eye Study. Ophthalmology. 2006;113(4):565-9. 45. Boonman ZF, de Keizer RJ, et al. Smoking delays the response to treatment in episcleritis and scleritis. Eye (Lond). 2005;19(9):949-55. 46. Klein BE, Klein R, Linton KL et al. Cigarette smoking and lens opacities: The Beaver Dam Eye Study. Am J Prev Med. 1993;9(1):27-30. 47. Theodoropoulou S, Theodossiadis P, Samoli E, et al. The epidemiology of cataract: a study in Greece. Acta Ophthalmol 2011;89(2):e167-73. 48. Ye J, He J, Wang C, et al. Smoking and risk of age-related cataract: a meta-analysis. Invest Ophthalmol Vis Sci. 2012;53:3885-95. 49. Wu R, Wang JJ, Mitchell P, et al. Smoking, socioeconomic factors, and age-related cataract: the Singapore Malay Eye Study. Arch Ophthalmol. 2010;128(8):1029-35. 50. Torp-Pedersen T, Boyd HA, Poulsen G, et al. In-utero exposure to smoking, alcohol, coffee, and tea and risk of strabismus. Am J Epidemiol. 2010(15);171(8):868-75. 51. Fernandes M, Yang X, Li JY, et al. Smoking during pregnancy and vision difficulties in children: a systematic review. Acta Ophthalmol. 2015;93(3)(5):213-23. 52. Xing L, Ye L, Zhu W, et al. Smoking was associated with poor response to intravenous steroids therapy in Graves’ ophthalmopathy. Br J Ophthalmol. 2015;99(12):1686-91. 53. Schwitzer T, Schwan R, Albuisson E, et al. Association between regular cannabis use and ganglion cell dysfunction. JAMA Ophthalmol. 2017;135(1):54-60. 54. Kamble G, Rani J, Taranikanti M, et al. Intraocular Pressure in smokers and nonsmokers. Niger J Physiol Sci. 2007;22(1-2):33-6. 55. Lee A, Rochtchina, E, Wang J, et al. Does smoking affect intraocular pressure? Findings from the blue mountain study. J Glaucoma. 2003;12(3):209-12. 56. Bonovas S, Filioussi K, Trasntes A, et al. Epidemiological association between cigarette smoking and primary open-angle glaucoma. Arch Ophthalmol 2003;121(12):256-61. 57. Jain V, Jain M, Abdull MM, et al. The association between cigarette smoking and primary open-angle glaucoma: a systematic review. Int Ophthalmol 2017;37:291-301. 58. Zanon-Moreno V, Garcia-Medina JJ, Zanon-Viguer V, et al. Smoking, an additional risk factor in elder women with primary open-angle glaucoma. Mol Vis. 2009;15:2953-9. 59. Dismuke WM, Ellis DZ. Activation of the BK(Ca) channel increases outflow facility and decreases trabecular meshwork cell volume. J Ocul Pharmacol Ther. 2009;25(4):309-14. 60. Ellis DZ, Dismuke WM, Chokshi BM. Characterization of soluble guanylate cyclase in NO induced increases in aqueous humor outflow facility and in the trabecular meshwork. Invest Ophthalmol Vis Sci. 2009;50(4):1808-13. 61. Marazita M, Dugour, Marquioni-Ramella M, et al. Oxidative stress-induced premature senescence dysregulates VEGF and CFH expression in retinal pigment epithelial cells: Implications for Age-related Macular Degeneration. Redox Biology. 2016;7:78-87. 62. Jabbarpoor Bonyadi MH, Yaseri M, Bonyadi M, Soheilian M, Nikkhah H. Association of combined cigarette smoking and ARMS2/LOC387715 A69S polymorphisms with age-related macular degeneration: A meta-analysis. Ophthalmic Genet. 2017;1:1-6. 63. Schmidt S, Hauser M, Scott W, et al. Cigarette smoking strongly modifies the association of LOC387715 and Age-related macular Degeneration. Am J Hum Genet. 2006;78(5):852-64 64. Thorne JE, Daniel E, Jabs DA, et al. Smoking as a risk factor for cystoid macular edema complicating intermediate uveitis. American journal of ophthalmology. 2008;145(5):841-6. 65. Franz CA, Frishman WH. Marijuana Use and Cardiovascular Disease. Cardiol Rev. 2016;24(7):158-62. 66. Panahi Y, Manayi A, Nikan M, et al. The arguments for and against cannabinoids application in glaucomatous retinopathy. Biomed Pharmacother. 2017:86(2);620-7. 67. Cairns EA, Toguri JT, Porter RF, et al. Seeing over the horizon -targeting the endocannabinoid system for the treatment of ocular disease. J Basic Clin Physiol Pharmacol. 2016; 27(3)(5):253-65. 68. Schweitzer KS, Chen SX, et al. Endothelial disruptive proinflammatory effects of nicotine and e-cigarette vapor exposures. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2015:309(7):L175-L187. 69. Kleinloog D, Liem-Moolenaar M, Jacobs G, et al. Does olanzapine inhibit the psychomimetic effects of Δ9-tetrahydrocannabinol. Journal of Psychopharmacology. 2012;26(10):1307-16. 70. Akano OF. Marijuana Use and Self-Reported Quality of Eyesight. Optom Vis Sci. 2017;94(5):630-3. 71. Bardak H, Bardak Y, Ercalik Y et al. Evaluation of the acute changes in objective accommodation, pupil size and ocular wavefront aberrations after cigarette smoking. Cutan Ocul Toxicol. 2017;36(1)(3):25-8. 72. Lerner AG, Goodman C, Rudinski D, et al. Benign and time-limited visual disturbances (flashbacks) in recent abstinent high-potency heavy cannabis smokers: a case series study. Isr J Psychiatry Relat Sci. 2011;48(1):25-9. 73. Schwitzer T, Schwan R, Laprevote V, et al. Transient Retinal Dysfunctions after Acute Cannabis Use. Eur Addict Res. 2016;22(6):287-91. 74. Russo EB, Merzuoki A, Mesa JM, et al. Cannabis improves night vision: A case study of dark adaptometry and scotopic sensitivity in kif smokers of the Rif mountains of northern Morocco. J Ethnopharmacol. 2004;93(1)(7):99-104. 75. Mantelli F, Lambiase A, Sacchetti M, et al. Cocaine snorting may induce ocular surface damage through corneal sensitivity impairment. Graefes Arch Clin Exp Ophthalmol. 2015;253(5):765-72. 76. Sánchez-Villarejo MV, López-Pedrajas R, Sánchez-Vallejo V, et al. Chronic cocaine effects in retinal metabolism and electrophysiology: treatment with topiramate. Curr Eye Res. 2014;39(5):493-503. 77. Nestler EJ. The Neurobiology of Cocaine Addiction. Science & Practice Perspectives. 2005;3(1):4-10. 78. Colatrella N, Daniel TE. Crack eye syndrome. J Am Optom Assoc. 1999;70(3):193-7. 79. Grzybowski A. Cocaine and the eye: a historical overview. Ophthalmologica. 2008;222(5):296-301. 80. Vasconcelos SBD, Guerra FM, Morato GM et al. Acquired anterior staphyloma after corneal ulcer associated with the use of crack. Arq. Bras. Oftalmol. 2016;79(7):4 81. Miller AD, Sherman SC. Crack eye. J Emerg Med. 2009;37(1):75-6. 82. Ravin JG, Ravin LC. Blindness due to illicit use of topical cocaine. Ann Ophthalmol. 1979;11(6):863-4. 83. Ghosheh FR, Ehlers JP, Ayres BD, et al. Corneal ulcers associated with aerosolized crack cocaine use. Cornea. 2007;26(8):966-9. 84. Lascaratos G, McHugh J, McCarthy K, Bunting H. Advanced cocaine-related necrotising sinusitis presenting with restrictive ophthalmolplegia. Orbit. 2016;35(3):164-6. 86. Siemerink MJ, Freling NJM, Saeed P. Chronic orbital inflammatory disease and optic neuropathy associated with long-term intranasal cocaine abuse: 2 cases and literature review. Orbit. 2017;36(5):350-5. 87. Silva-Araújo A, Tavares MA. Development of the eye after gestational exposure to cocaine. Vascular disruption in the retina of rats and humans. Ann N Y Acad Sci. 1996;801:274-88. 88. Tran KH, Ilsen PF. Peripheral retinal neovascularization in talc retinopathy. Optometry. 2007;78(8):409-14. 89. Zoumalan C, Marmor M. Revisiting Talc Retinopathy. Arch Ophthalmol. 2007;125(7):988. 90. Stefano GB, Pilonis N, Ptacek R, Kream RM. Reciprocal Evolution of Opiate Science from Medical and Cultural Perspectives. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research. 2017;23(6):2890-6. 91. Weiss RD, Rao V. The Prescription Opioid Addiction Treatment Study: What have we learned. Drug and Alcohol Dependence. 2017;173(4):S48-S54. 92. Martin-Moro JG, Calleja JML, Garcia MB, et al. Rhinoorbitocerebral mucormycosis: A case report and literature review. Med Oral Patol Oral Cir Bucal. 2008;13(12):E792-5. 93. Doan T, Vemulakonda GA, Choi D, et al. Retinal Neovascularization and Endogenous Fungal Endophthalmitis in Intravenous Drug Users. Ophthalmology. 2014;121(9):1847-8 94. Bagheri N, Shahlaee A, Sridhar J, Ho AC, En face optical coherence tomography and angiography of talc retinopathy. Acta Ophthalmol. 94(1):103–4. 95. Patel SN, Rescigno RJ, Zarbin MA, et al. Endogenous endophthalmitis associated with intravenous drug abuse. Retina. 2014;34(7):1460-5 96. Malecaze F, Arne JL, Bec P, et al. Candida endophthalmitis after heroin abuse. Mycopathologia. 1985;92(2):73-6. 97. Bettendorf BA, Thomson M, Reichstein D, Thomas J. Acute central vision loss in an IV drug use. Poster presented at Midwest Society of General Internal Medicine Meeting. Sept 13, 2013; Chicago, IL. 99. Rabin RL. A Case Report of Nystagmus with Acute Comitant Esotropia Secondary to Heroin Withdrawal: A Novel Presentation. Case Reports in Ophthalmology. 2015;6(3):333-8. 100. Firth AY. Heroin and diplopia. Addiction. 2005;100(1):46-50. 101. Pickworth WB, Welch P, Henningfield JE, Cone EJ. Opiate-induced pupillary effects in humans. Methods Find Exp Clin Pharmacol. 1989;11(12):759-63. 102. Kocherlakota P. Neonatal abstinence syndrome. Pediatrics. 2014;134(2):e547-e561 103. Spiteri Cornish K, Hrabovsky M, Scott NW, et al. The short- and long-term effects on the visual system of children following exposure to maternal substance misuse in pregnancy. Am J Ophthalmol. 2013;156(1):190-4. 104. Gill AC, Oei J, Lewis NL, et al. Strabismus in infants of opiate-dependent mothers. Acta Paediatr. 2003;92(3):379-85. 105. Hamilton R, McGlone L, MacKinnon JR, et al. Ophthalmic, clinical and visual electrophysiological findings in children born to mothers prescribed substitute methadone in pregnancy. Br J Ophthalmol. 2010;94(6):696-700. 106. Hazin R, Cadet JL, Kahook MY, Saed D. Ocular manifestations of crystal methamphetamine use. Neurotox Res. 2009;15(2):187-91. 107. Wijaya J, Salu P, Leblanc A, Bervoets S. Acute unilateral visual loss due to a single intranasal methamphetamine abuse. Bull Soc Belge Ophtalmol. 1999;271:19-25. 108. Charukamnoetkanok P, Wagoner MD. Facial and ocular injuries associated with methamphetamine production accidents. Am J Ophthalmol. 2004;138(5):875-6. 109. Lai H, Zeng H, Zhang C, et al. Toxic Effect of Methamphetamine on the Retina of CD1 Mice. Current eye research. 2009;34(9):785-90. 110. Poulsen EJ, Mannis MJ, Chang SD. Keratitis in methamphetamine abusers. Cornea. 1996;15(9):477-82. 111. Wallace RT, Brown GC, Benson W, Sivalingham A. Sudden retinal manifestations of intranasal cocaine and methamphetamine abuse. Am J Ophthalmol. 1992;114(2):158-60. 112. Semeraro F, Forbice E, Romano V. Neurotrophic keratitis. Ophthalmologica. 2014;231(4):191-7. 113. The ASAM Criteria. Resources. American Society of Addiction Medication. www.asam.org/resources/the-asam-criteria/about. Accessed April 26, 2018. |

