 A 61-year-old white female had been followed in my office for several years as a glaucoma suspect after she initially presented in 2000 with suspicious neuroretinal rims. She had no known history of glaucoma. Her medications include Zocor (simvastatin, Merck) q.d. and 81mg of aspirin q.d. She reported no known allergies to medications.
A 61-year-old white female had been followed in my office for several years as a glaucoma suspect after she initially presented in 2000 with suspicious neuroretinal rims. She had no known history of glaucoma. Her medications include Zocor (simvastatin, Merck) q.d. and 81mg of aspirin q.d. She reported no known allergies to medications.
Diagnostic Data
Her best-corrected visual acuity was 20/20 O.D., O.S. and O.U. through myopic astigmatic and presbyopic correction. Pupils were equal, round and reactive to light and accommodation, with no afferent pupillary defect. Her extraocular motilities were full in all positions of gaze.
Her cup-to-disc measurements of 0.60 x 0.70 O.D. and 0.50 x 0.55 O.S. were of significance at the initial evaluation. Her right neuroretinal rim was thinned at the 7 oclock position, and there was a moderate amount of peripapillary atrophy in the same region. The right nerve characteristics did not follow the inferior-superior-nasal-temporal (ISNT) rule. While a mild amount of peripapillary atrophy was present O.S., the rim looked healthy.
The patient was followed for many years with intraocular pressure measurements that averaged 14mm Hg to 17mm Hg O.D. and 14mm Hg to18mm Hg O.S. Pachymetry readings were 503m O.D. and 519m O.S. She was a reliable visual field taker, and all fields were completely normal. Gonioscopy demonstrated grade 4+ open angles O.U., with minimal trabecular pigmentation and no angle abnormalities.
A slit lamp examination of her anterior segments was consistently unremarkable, as was her retinal examination, which demonstrated clear maculae, mild arteriolar sclerotic retinopathy O.U. and clear peripheral retinal grounds. She had a posterior vitreous detachment O.D. and floaters O.S.
In early 2005, there appeared to be early changes to her neuroretinal rim O.D., as was determined by stereo photography and stereo photo overlay software. The left optic nerve remained stable. We recorded a few IOP readings in the 18mm Hg to 21mm Hg range O.D.; however, her measurements remained the same O.S. Visual fields remained stable O.U. While the changes O.D. were subtle and gradual, they were repeatable upon examination over time. By the fall of 2005, I believed that the patient was inching toward glaucomatous optic neuropathy O.D. So, I initiated therapy in her right eye only.
In 2007, the patients left eye began to exhibit a similar transition. We noted a slight IOP increase of 1mm Hg to 3mm Hg O.S.
She also demonstrated subtle changes to the left neuroretinal rim, and we began medication in the left eye as well.
Discussion
This case is a prime example of a subtle conversion of a glaucoma suspect to glaucoma patientin this case, a pre-perimetric normal tension glaucoma patient.
Conversion from a state that simply requires passive monitoring to a state that requires active therapy may be precipitated by a consistent rise in IOP, glaucomatous visual field loss and/or changes in the neuroretinal rim. In this case, the conversion manifested as progressive changes in her neuroretinal rims in the absence of visual field loss.
It is safe to say that our patient clearly did convert to a glaucomatous situation because of documented changes to her neuroretinal rims. But, the question remains: Is she now stable?
Clouding the issue is the fact that her post-treatment IOP is, in fact, very similar to her pre-treatment IOP. The topic of setting target IOP has been discussed previously in this column; however, it is important to keep in mind that target IOPs are dynamic figures that need to be reassessed and readjusted periodically, depending on other physical criteria, such as neuroretinal rim characteristics, changes in angle anatomy or visual field changes. In this patients conversion, we assume that IOP fluctuations may have played a significant role. Subsequently, it is paramount to maintain a tight range of post-treatment IOP to prevent more damage.
Our patients post-treatment IOP readings have consistently varied by no more than 3mm Hg in either eye, but does that guarantee stability? No. This is because IOP changes may occur at times other than during office hours.1
So how do we know this patient is truly stable? To be sure, we need to look very carefully at those same measurements we looked at before she convertednamely, IOP, visual fields and optic nerve characteristics. We need to keep in mind that her initial conversion was manifested by changes in her neuroretinal rim.
So, it is important that we look very carefully at the neuroretinal rim moving forward, as that is where she is most likely to exhibit further deterioration.
One outstanding tool that is used to evaluate neuroretinal rim stability is the Topographic Change Analysis (TCA), which is generated by the Heidelberg Retinal Tomographer (HRT). This analysis provides a precise look at neuroretinal rim area and volume over time. Any decreases in neuroretinal rim area or volume are clearly shown as red flagged areas, which are easy to follow over time.
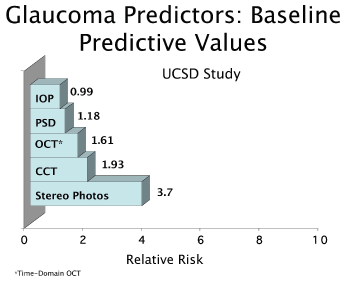
1. Simple stereo photography is one of the most effective ways to predict glaucoma risk.
Incorporating advanced technology into your office, such as HRT, is a decision that each of us has to make in terms of what is best for our patients, what is best for our practices, and what is best for assisting us in the treatment of ocular diseases. While the technology is wonderful, it is a bit pricey, and a cost analysis needs to be done prior to purchasing such equipment.
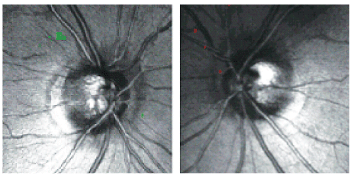
2. At our patients most recent visit on July 21, 2008, the Topographic Change Analysis images demonstrate no significant changes in her neuroretinal rim characteristics.
If you recently started your office or do not yet have the patient base to pay for this technology, dont worry. In the context of glaucoma, research has demonstrated that simple stereo photography is far more powerful in predicting glaucoma than IOP, central corneal thickness, visual fields and OCT technology (figure 1).2
Considering, this, you may want to equip the new office with a digital stereo fundus camera first, and when finances permit, add more advanced technology, such as the HRT-3.
As for our patient, she now takes one drop of Travatan Z (travoprost, Alcon) O.U. h.s. Her right eye has been medicated for three years and her left eye for about 18 months. Her current cup-to-disc ratios are 0.70 x 0.80 O.D. and 0.60 x 0.65 O.S. Post-treatment IOP has averaged 15mm Hg O.D. and 14mm Hg O.S. Her visual fields have remained stable and unremarkable (figure 2).
1. Asrani S, Zeimer R, Wilensky J, et al. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J Glaucoma 2000 Apr;9 (2):134-42.
2. Lalezary M, Medeiros FA, Weinreb RN, et al. Baseline optical coherence tomography predicts the development of glaucomatous change in glaucoma suspects. Am J Ophthalmol 2006 Oct;142(4):576-82.

