Contact lenses are a medical marvel that, in today’s world, is often taken for granted. First made from blown glass in the late 1800s, contact lenses have progressed significantly over the last 120 years. With every advancement in contact lens technology, access to contact lens wear has increased exponentially, but despite that, reactions have become somewhat ho-hum.
While patients who require contacts for medical purposes appreciate their life-changing benefits, many of our day-to-day patients are no longer astounded by the promise and impact of a little piece of plastic on their eye that helps them see. But little do they realize how their vision relies on the relationship between these medical marvels and the tear film.
This article will discuss the interactions of contact lenses with the tear film, how suboptimal contact lens-tear film interactions can impact contact lens wear and various management strategies to improve and optimize that interaction.
Inside the Layers
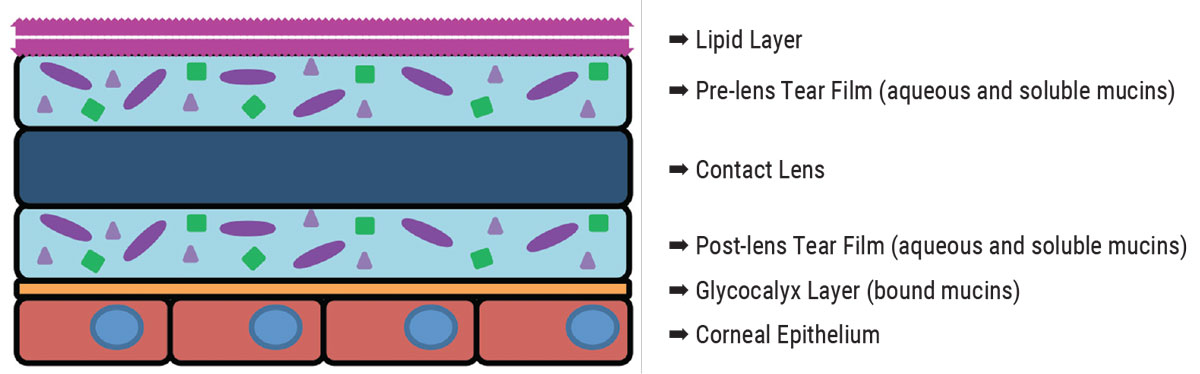
From a patient’s perspective, successful contact lens wear depends on vision and comfort, and nothing plays a greater role in promoting success than the tear film. The tear film consists of an outer oil layer produced by the meibomian glands of the eyelids that prevents evaporation of the aqueous portion of the tear film. Below the lipid layer exists a mixture of aqueous fluid produced by the main and accessory lacrimal glands and soluble mucins produced by conjunctival goblet cells that hydrate and lubricate the ocular surface.
The innermost layer is the glycocalyx, which consists of insoluble mucins produced by conjunctival goblet cells and assists in adhering the tear film to the corneal epithelium.1 When a contact lens is applied to the eye, it splits the tear film into two halves: a pre-lens tear film and a post-lens tear film (Figure 1).2-5
 |
|
Fig. 1. A contact lens splits the tear film into pre-lens and post-lens layers with different components. Click image to enlarge. |
The pre-lens tear film consists of the outer lipid layer of the tear film and a portion of the aqueous and mucin mixture.2,4,5 It covers the outer surface of the lens and provides the smooth optical surface required for vision as well as a lubrication layer between the contact lens and the palpebral conjunctiva of the eyelids.
The post-lens tear film consists of the remaining portion of the aqueous and mucin mixture and the glycocalyx layer, providing lubrication and a cushion between the contact lens and cornea and bulbar conjunctiva.2,4,5
For an individual with a normal tear film, there is adequate volume to both the pre-lens and post-lens tear films such that they do not become destabilized when divided by the contact lens. This leads to good quality, stable vision and good comfort, resulting in successful contact lens wear.
Contrary to that, an individual who is deficient in one or multiple of these tear film components may struggle with contact lens wear due to poor vision, comfort or both.
Common Complications
Every eye care provider likely has at least one patient per day who complains of blurry, fluctuating vision that changes constantly as they blink. And many can probably think of several patients who have this complaint specifically with wearing their contact lenses. Visual instability like this may be the result of an unstable lipid layer leading to more rapid evaporation of the tear film, or it may be due to poor lens surface wetting. Patients with rapid tear break-up time typically suffer from various lid conditions such as meibomian gland dysfunction or blepharitis, and management of those conditions should be targeted to improve their symptoms.
In the absence of some pathology, poor surface wetting of contact lenses can develop secondary to lens deposits, which may be organic or inorganic in nature. Common organic deposits include lipids and proteins commonly found in the tear film. Common inorganic deposits include contamination from mascara, hairspray, lotions and soaps.6
With the increased use of daily disposable lenses, surface deposits have become far less common, but when they are encountered, remind patients to rub their lenses and thoroughly wash their hands prior to handling the lens or recommend a different cleaning regimen such as a surfactant or enzymatic cleaner. Enforcing these steps can make all the difference.6
For gas permeable (GP)-style contact lenses, including corneal and scleral modalities, maintaining excellent surface wetting can sometimes be a challenge. The materials used to create theses lenses are typically hydrophobic in nature, thus care regimens for GP-style modalities involve both cleaning and conditioning the lens to promote lens surface wettability.7 Surface contamination by various cosmetic products can greatly reduce surface wettability and may require thorough rubbing to remove or the use of a strong laboratory-style cleaner.6
To help promote initial surface wetting, the lens manufacturer can do plasma treatment, which removes a thin layer of organic matter present on the surface of the lens producing a hyper-clean surface.6 The removal of this layer of organic matter helps to improve the surface wettability of these lenses.
Practitioners can also request to have Tangible Science’s Hydra-PEG coating added to lenses, which is a hydrophilic layer chemically bonded to the surface of GP lenses, promoting tear film adherence to the lens surface. Hydra-PEG has been a game-changer and has become a first-line treatment strategy for GP contact lens wearers suffering from issues associated with surface wettability.
Another common issue practitioners encounter is contact lens discomfort. Patients who are asymptomatic without contact lens wear may complain of grittiness, dryness, scratching, irritation, redness or myriad other symptoms when wearing contact lenses.4,8-10
The tear film provides lubrication between the contact lens and the ocular surfaces it interacts with, including the palpebral conjunctiva lining the eyelids, the bulbar conjunctiva covering the sclera and the cornea itself. Without lubrication, these tissues are directly exposed to the harsh mechanical interaction of the contact lens as it moves during blinking.4,8-10 The palpebral conjunctiva rubs across the surface of the contact lens rather than gliding. This can lead to the development of papillary and/or giant papillary conjunctivitis as well as keratinization of the palpebral conjunctiva along the lid margin, known as lid wiper epitheliopathy (LWE).3,11 The bulbar conjunctiva can be affected similarly. Without adequate tear film volume, a contact lens’s movement is limited or may not move at all. This in turns leads to irritation and inflammation of the ocular surface, which decreases patient comfort.
Adjusting a lens parameter such as the base curve or diameter of a soft contact lens or the landing zone of a scleral or hybrid contact lens to loosen the fit of the lens may be enough to remedy this issue.6,7 Otherwise, ensuring adequate tear volume is present provides a cushion for the contact lens to rest on rather than directly bearing on the cornea or conjucntiva. The cornea and conjunctiva are both very highly innervated tissues, and slight changes in pressure, temperature and pain can be readily detected.1,3,4 Therefore, reducing or eliminating any extra pressure or pain stimulation of those nerves is paramount to contact lens wearing success.
Neophyte Evaluation
When examining a new patient who desires contact lenses, start with a thorough ocular health evaluation for any signs of meibomian gland dysfunction, blepharitis, reduced tear break-up time or low tear volume. More advanced testing such as non-invasive tear break-up time, lipid layer analysis, meibography, blink analysis, tear osmolarity and InflammaDry (Quidel) can help further identify these potential troublemakers and provide guidance for a targeted treatment approach.
For patients who are looking to begin wearing contact lenses, identifying and treating these conditions prior to lens wear can increase their success.12
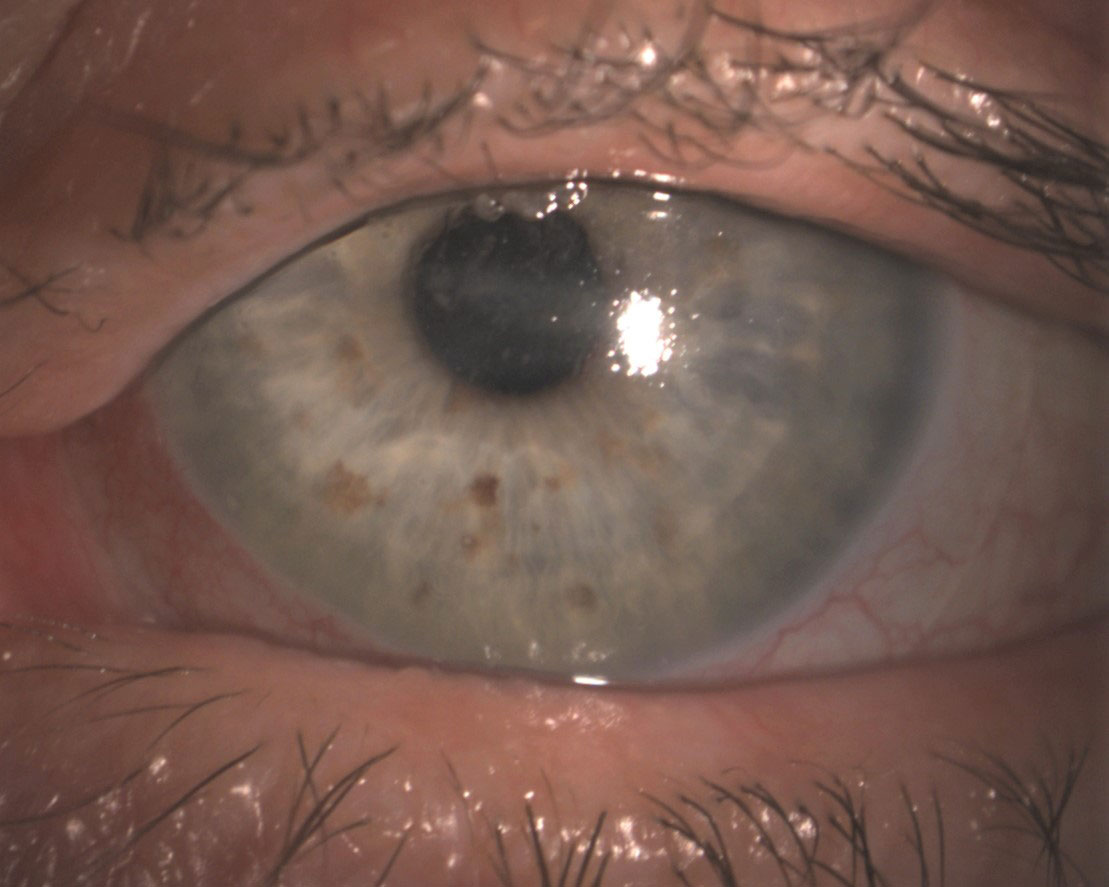
For new contact lens wearers and those returning for their annual evaluations, it is imperative to assess the ocular surface both during and after contact lens wear. Begin the assessment with white light to evaluate the contact lens fit for movement, lens centration and surface wetting (Figure 2). Also, evaluate the lid margin for signs of meibomian gland dysfunction, tear film debris and signs of ocular surface irritation such as injection or corneal infiltrates.
 |
|
Fig. 2. A scleral lens evaluated with white light that demonstrates poor surface wetting and mucus build-up on a patient with neurotrophic keratitis. Click image to enlarge. |
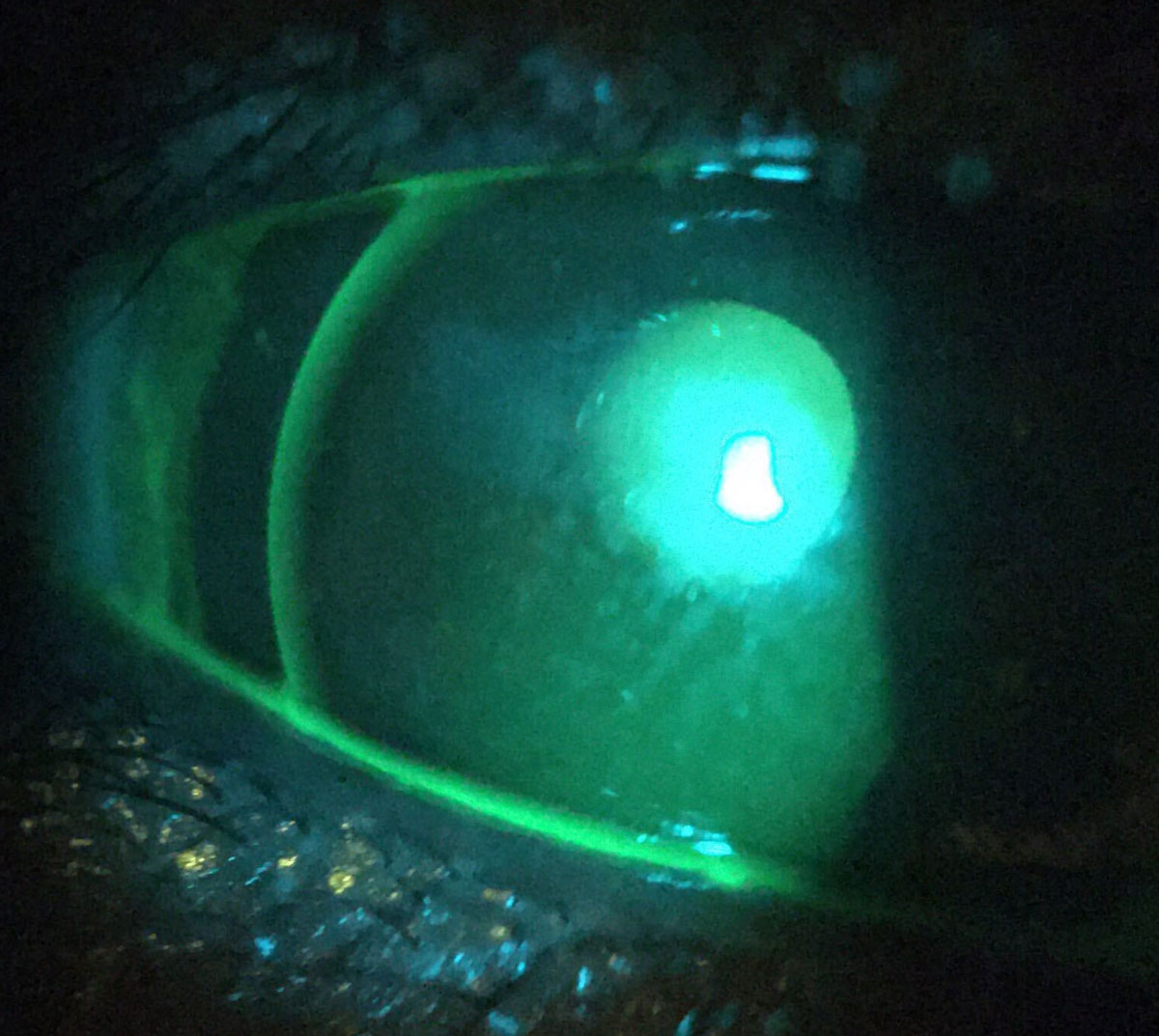
For GP and hybrid lenses, apply sodium fluorescein to the ocular surface to assess the fit of the contact lens prior to removal. By staining the tear film, the interaction of the contact lens and the ocular surface can be more directly visualized. It also makes it possible to visualize the surface wettability of GP lenses more directly (Figure 3). This can also be done with silicone hydrogel (SiHy) soft contact lenses but not hydrogel lenses, as the fluorescein can be absorbed into the matrix of those lenses.
 |
|
Fig. 3. Sodium fluorescein staining demonstrates front surface tear film break-up in this GP lens patient. Click image to enlarge. |
Following removal of the contact lenses, use both lissamine green and sodium fluorescein vital dyes to thoroughly evaluate the ocular surfaces. Lissamine green provides excellent visualization of the conjunctiva and highlights areas of irritation associated with contact lens wear, such as circumferential staining around the cornea that can result from a contact lens that is too tight.
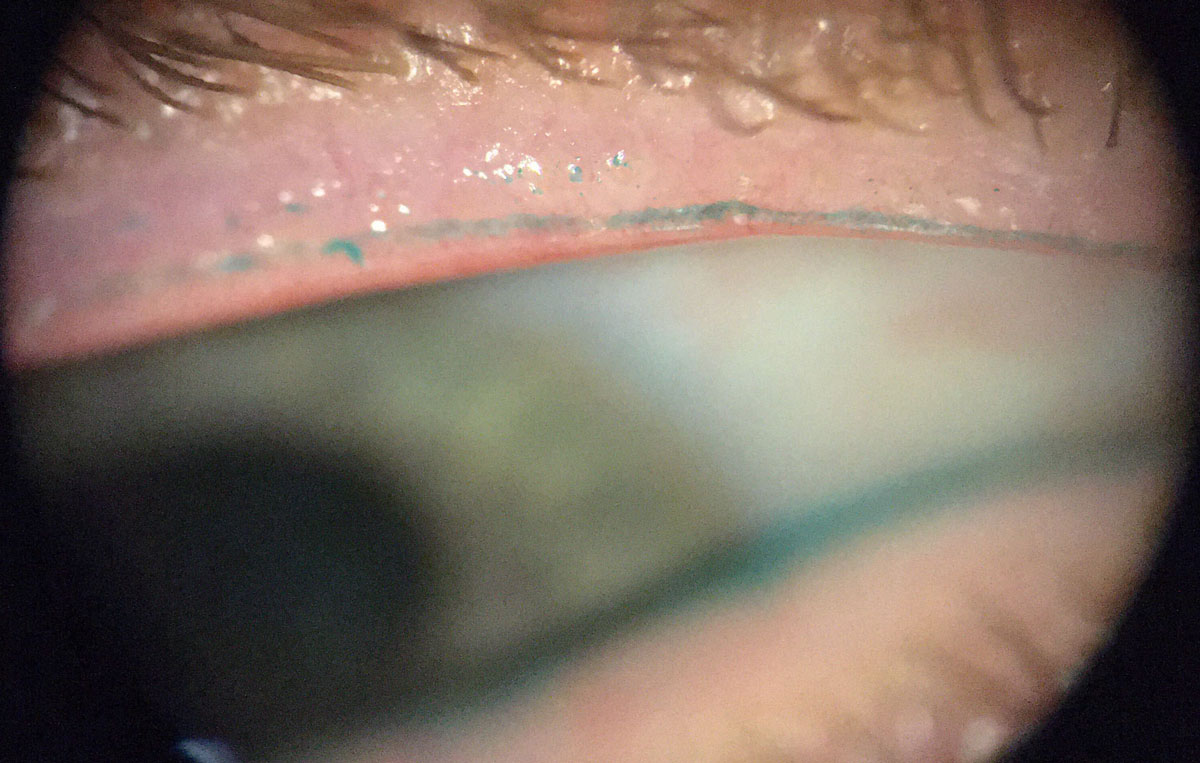
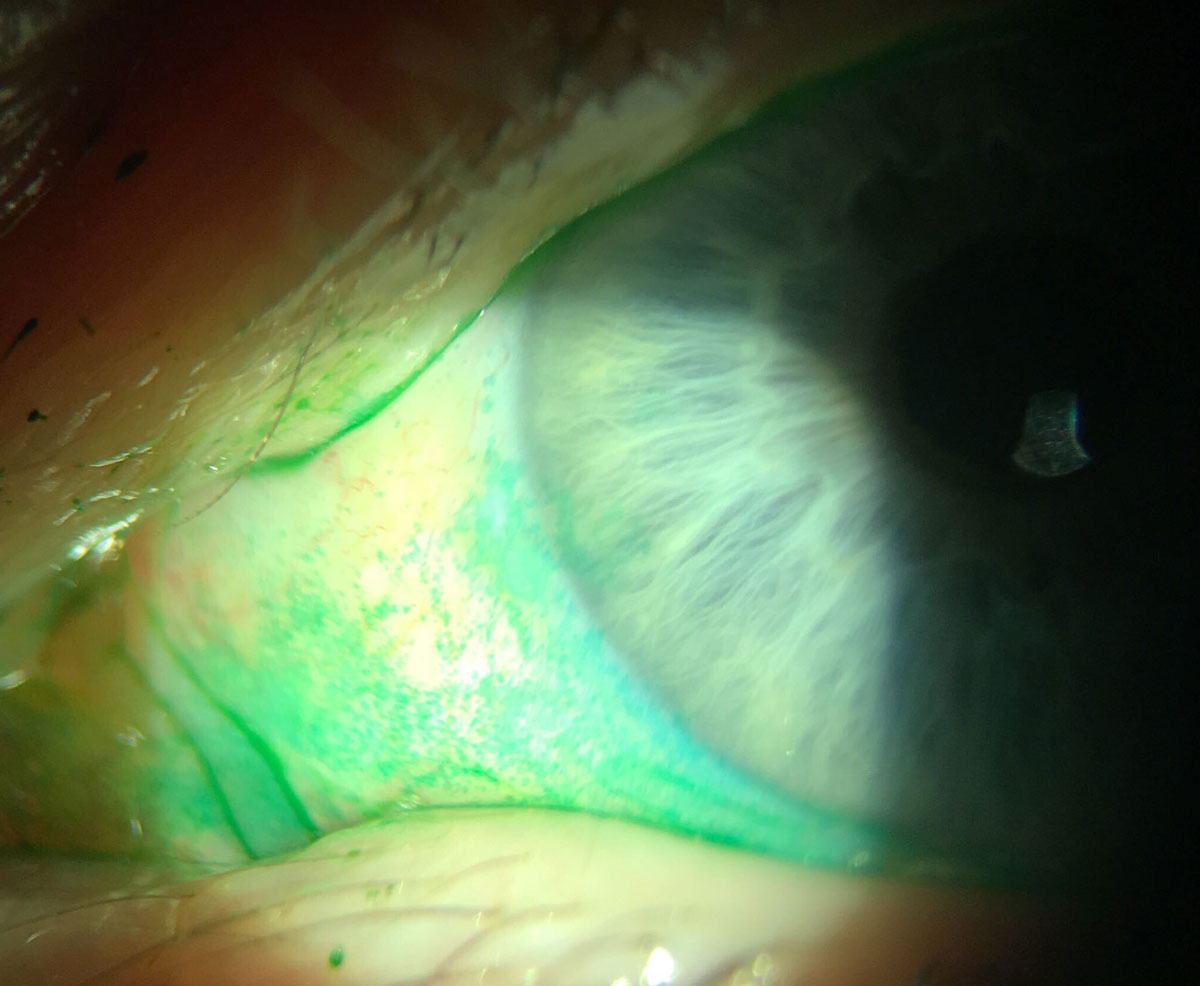
Staining is also needed for visualizing LWE on the palpebral conjunctiva (Figure 4). Typically, there should be a line of staining at the mucocutaneous junction, known as the line of Marx; any staining of the adjacent palpebral conjunctiva beyond that is evidence of LWE.8,9
 |
|
Fig. 4. Bulbar conjunctival staining with lissamine green (bottom) and palpebral conjunctival staining (top) characteristic of mild LWE. Click image to enlarge. |
 |
The lid wiper normally contacts the ocular surface and contact lens and acts as a squeegee to distribute the tears, but as the tissue becomes keratinized, it can no longer distribute the tears across the ocular surface as easily or efficiently.
Imagine a car whose windshield wipers are moving back and forth without any windshield wiper fluid. Rather than gentle gliding across the windshield, the wiper blades streak and stutter across, resulting in damage to the wipers and poor cleaning of the windshield. The same effect happens as the lids blink across a poorly wetting contact lens, which can develop in both soft and GP contact lens wearers alike. This results in increased friction, which further exacerbates the epitheliopathy and worsens patient symptoms (e.g., foreign body sensation, grittiness, irritation).11
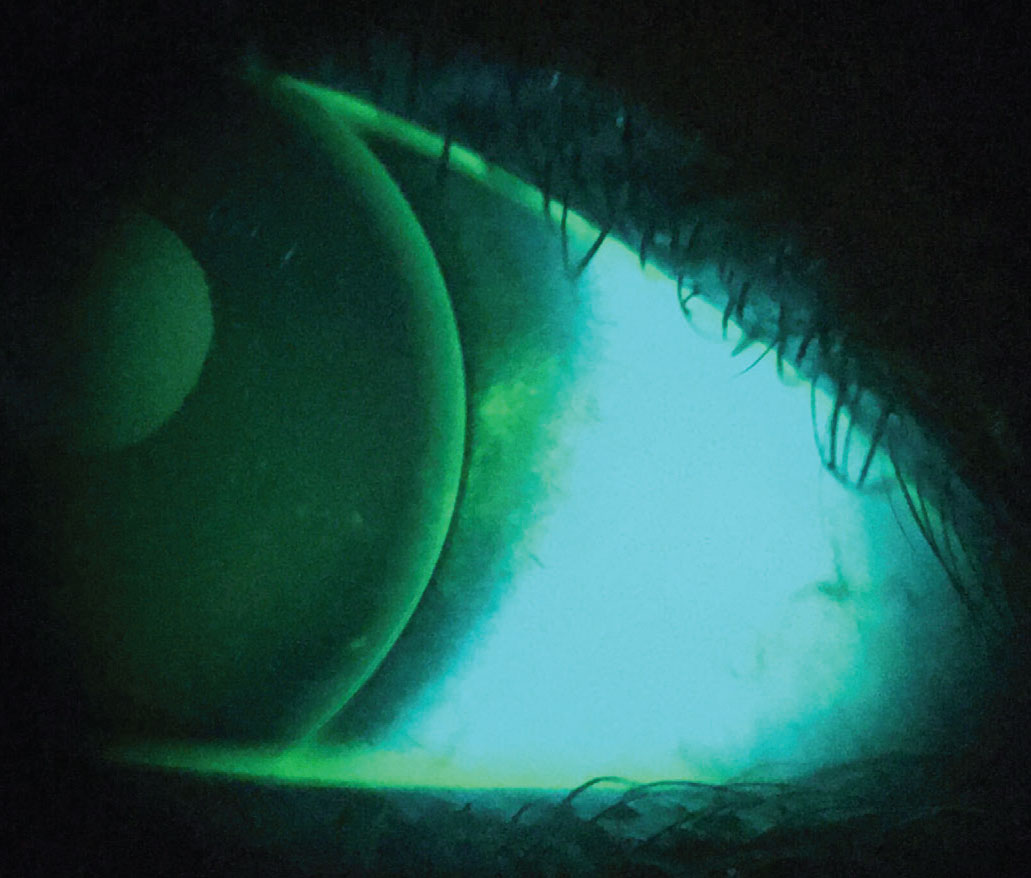
Sodium fluorescein can stain dead epithelial cells on both the cornea and conjunctiva, highlighting areas of compromised integrity of the ocular surface, which results from both contact lens wear and dry eye.12 The presence of this staining is a good indicator of ocular surface inflammation related to dry eye but can also be the result of increased friction as the contact lens moves across the cornea or conjunctiva. Areas that become dehydrated during contact lens wear can also develop these points of staining, a common example of this being 3 and 9 o’clock staining that can be seen with corneal GP lenses (Figure 5). By providing direct visualization of the tear film, fluorescein allows for evaluation of tear film break-up time and tear meniscus height. In cases of GP, hybrid and SiHy lens modalities, by assessing this staining both during and after contact lens wear, one is able to evaluate the impact a contact lens has on the tear film dynamics.
 |
|
Fig. 5. Punctate staining present along the edge of a corneal GP lens characteristic of “3 and 9 o’clock” staining. Click image to enlarge. |
Treat Underlying Conditions
For patients with underlying conditions such as meibomian gland dysfunction or dry eye disease, management of those conditions should be targeted first. For meibomian gland dysfunction, there are typically two different levels of treatment: at-home thermal therapy, which use various heat masks to improve or maintain gland function, or in-office thermal pulsation therapies, which combine thermal treatment with mechanical pulsation to more fully express the meibomian glands.
Improving meibomian gland function helps increase the lipid layer of the tear film and promotes tear film stability. For patients who have significant meibomian gland dropout, supplementation with a lipid-based artificial tear may also be beneficial.
Dry eye disease may present in a myriad of ways and often requires multiple treatment strategies. To manage ocular surface inflammation, topical immune modulators, such as Restasis (cyclosporine A emulsion 0.05%, Allergan), Xiidra (lifitegrast ophthalmic solution 5%, Novartis) and Cequa (cyclosporine ophthalmic solution 0.09%, Sun Ophthalmics), are commonly used.13
For patients with reduced tear volume, techniques to increase it can be targeted towards supplementation, decreasing tear outflow or stimulating tear production. Increasing tear volume via artificial tear supplementation can be effective but tedious. Some patients may only require two or three drops throughout the day, while others may require one drop per hour to maintain comfort and vision. When using artificial tears more than four times per day, I recommend patients use preservative-free artificial tears.
For those patients requiring several supplemental drops throughout the day, punctal plugs can help reduce or eliminate the need for artificial tear drops by blocking the nasolacrimal drainage system. Increasing the production of the natural tear film components is another great method. Various oral medications, such as cevimeline and pilocarpine, and topical medications, such as diquafosol and rebamipide, exist that increase tear production and may be indicated based on the severity of the patient’s condition.2,5,9,10,14
Once other underlying diseases are managed in patients still struggling with contact lens wear, consider changing the contact lens parameters or modality. Standard soft contact lenses provide limited variation and often require changing brands to obtain a different base curve or diameter that is more desirable. For frequent-replacement soft lens modalities, reviewing and recommending care systems that reduce or eliminate exposure to preservatives can help improve patient comfort. Switching patients to daily disposable lenses can help improve patient comfort by reducing the tear film interference as well as providing a fresh lens every day for wear.
For custom soft and GP lens modalities there is a lot of freedom to adjust lens parameters such as base curve, diameter, optic zone size, peripheral curves and material that may be altered to optimize the contact lens fit and patient comfort. The addition of different lens treatments (e.g., plasma treatment) or coatings (e.g., Hydra-PEG) can also improve lens wettability and patient comfort.
For some trickier patients, sometimes a change in modality is required. Two of my favorite options are orthokeratology (ortho-K) and scleral contact lenses. Ortho-K presents an excellent correction option for myopic patients, especially those who report issues of dryness when wearing their contact lenses during the day but not their glasses. By wearing the ortho-K lenses at night in a closed- eye system, tear film evaporation is reduced and there is less interaction between the lens and eyelids. For those patients who are not candidates for or are not interested in ortho-K, scleral lenses provide another correction option.
Scleral lenses have become a mainstay treatment for chronic, severe dry eye patients but can also provide symptomatic relief and excellent comfort for patients who are otherwise intolerant to contact lens wear. Oftentimes, I will have patients return for an in-office scleral lens trial, where we place lenses on their eye and they can experience them in-office. Many patients appreciate the extra hydration and comfort of the lenses even during this short trial period in the office and choose to pursue scleral lenses.
Takeaways
For patients struggling to wear contact lenses, identification and management of any underlying conditions will greatly improve patient success and help keep them in their contact lenses. Once other causes have been ruled out, evaluation of the lenses and how they impact the ocular surface can help to direct what changes need to be made to help improve patient comfort. Some cases may require larger changes, such as switching to a different modality.
Whatever the situation, identifying your patient’s issue, explaining it to them, providing them with a targeted treatment plan, and delivering on that plan can build their confidence in you and keep them wearing contact lenses longer.
Dr. Fosso is the director of contact lens services at PineCone Vision Center in Sartell, MN. He is a fellow of the American Academy of Optometry as well as the Scleral Lens Society. He has consulted and lectured for the STAPLE Program, Euclid Systems Corp. and Valley Contax.
1. Remington LA. Ocular adnexa and lacrimal system. In: Clinical Anatomy and Physiology of the Visual System. 3rd ed. St. Louis: Elsevier Butterworth Heinemann; 2012:159-81. 2. Nichols JJ, Willcox MDP, Bron AJ, et al. The TFOS Interactional Workshop on Contact Lens Discomfort: executive summary. Invest Ophthalmol Vis Sci. 2013;54(11):TFOS7-13. 3. Efron N, Jones L, Bron AJ, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the contact lens interactions with the ocular surface and adnexa subcommittee. Invest Ophthalmol Vis Sci. 2013;54(11):TFOS98-122. 4. Craig JP, Wilcox MDP, Argüeso P, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the contact lens interactions with the tear film subcommittee. Invest Ophthalmol Vis Sci. 2013;54(11):TFOS123-56. 5. Kojima T. Contact lens-associated dry eye disease: recent advances worldwide and in Japan. Invest Ophthalmol Vis Sci. 2018;59(14):DES102-8. 6. Bennett ES, Henry VA. Soft lens care and patient education. In: Clinical Manual of Contact Lenses. 4th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins;2014:294-7. 7. Bennett ES, Henry VA. Gas-permeable lens care and patient education. In: Clinical manual of contact lenses. 4th ed. Philadlephia: Wolters Kluwer/Lippincott Williams & Wilkins;2014:157-61. 8. Jones L, Brennan NA, González-Méijome J, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the contact lens materials, design and care subcommittee. Invest Ophthalmol Vis Sci. 2013;54(11):TFOS37-70. 9. Chen Q, Wang J, Shen M, et al. Lower volumes of tear menisci in contact lens wearers with dry eye symptoms. Invest Ophthalmol Vis Sci. 2009;50(7):3159-63. 10. Chen Q, Wang J, Shen M, et al. Tear menisci and ocular discomfort during daily contact lens wear in symptomatic wearers. Invest Ophthalmol Vis Sci. 2011;52(5):2175-80. 11. Lievens C, Roberts L, Rayborn E, et al. Lid wiper epitheliopathy: what the OD needs to know. Rev Optom. 2020;157(11):62-8. 12. Bennett ES, Henry VA. Preliminary Evaluation. In: Clinical manual of contact lenses. 4th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins;2014:9-18. 13. Gann D, Nichols JJ. 2020 report on dry eye diseases. CL Spectrum. 2020;35(7):24-31,55. 14. Koh S. Contact lens wear and dry: beyond the known. Asia Pac J Ophthalmol (Phila). 2020;9(6):498-504. |

