 |
Open-angle glaucoma (OAG) or ocular hypertension management in those with corneal endothelial disease or in those who have undergone full- or partial-thickness keratoplasty, progressive optic nerve damage as well as progression of corneal disease or graft-related complications can lead to further visual decline. It’s valuable to review the nuances in managing intraocular pressure (IOP) with the goal of slowing vision loss due to OAG while being mindful of the potential impact of treatment on the cornea.
Two recent cases with an established diagnosis of severe stage OAG and significant concomitant corneal disease have highlighted the awareness needed in treatment considerations and the potential impact of treatment for each respective disease process on the progression of the other.
A 68-year-old man with history of corneal decompensation following phacoemulsification with supraciliary microstent implantation and device trim in the left eye presented for evaluation of OAG. His IOPs were 18mm Hg in each eye with central corneal thickness (CCT) of 582µm in the right eye and 633µm in the left eye. He had been using timolol 0.5% BID OU, Muro 128 QID OS and prednisolone acetate BID OS and had advanced glaucomatous optic neuropathy with significant functional loss in line with optic disc appearance.
The second case, a 72-year-old pseudophakic woman with history of penetrating keratoplasty (PK) more than 20 years prior for reported treatment of Salzmann nodular degeneration of the right eye, had IOPs of 32mm Hg OD and 14mm Hg OS with central corneal thickness of 518µm in the right eye and 511µm in the left eye at her initial visit. Her optic discs were obliquely inserted, consistent with increased axial length and previous high myopia with asymmetric but bilateral inferior neuroretinal rim loss. She reported a history of latanoprost, dorzolamide-timolol and brimonidine use without consistent adherence to any topical therapy and had self-discontinued all treatment about six weeks prior to presentation.
Surgical procedures that lower IOP in individuals with refractory OAG result in endothelial cell loss, which in rare cases may be progressive and necessitate either partial- or full-thickness corneal transplantation, and corneal transplantation is a risk factor for the development of elevated IOP, as well as the development or progression of glaucoma.
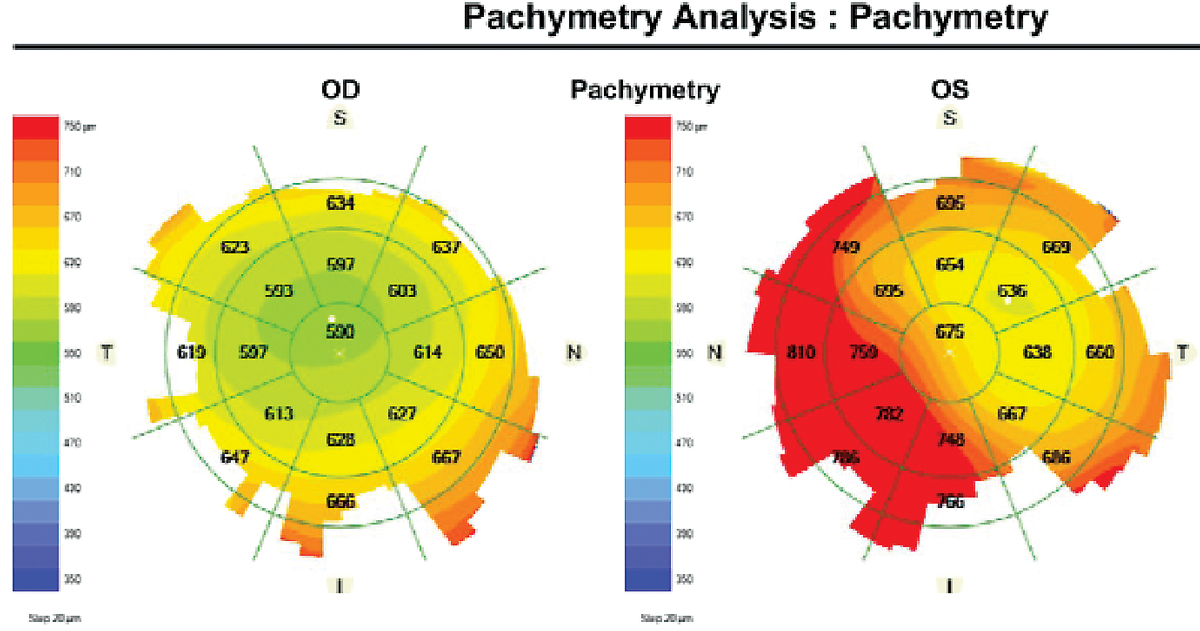 |
|
Increased corneal thickness in the left eye with corneal decompensation. Click image to enlarge. |
Before and After
Many continue to explore the impact of altering aqueous humor dynamics on ocular structures, including corneal endothelial cells in eyes with OAG through medical and surgical treatment.1 Endothelial cell count reduction is an expected occurrence after any intraocular procedure, including cataract surgery and vitreoretinal surgery; however, endothelial cell loss months to years after a procedure is an uncommon, but possible, event following glaucoma surgical procedures.2-4
The average incidence of chronic stromal edema five years postoperatively in one study was 11% and 12%, and the progressive endothelial cell density loss identified five years following supraciliary microstent insertion, which ultimately led to voluntary withdrawal followed by FDA Class 1 recall in 2018, supports the long-term need in monitoring corneal health in patients who undergo surgical procedures in the management of glaucoma.1-4 If corneal edema is present and CCT is increased, keep in mind the general overestimation of intraocular pressure by conventional tonometry measures.5 Corneal biomechanics are highly complex, and the impact of CCT on IOP is not accurately reflected by existing nomograms or correction factors based on central corneal thickness alone.
History of PK and lamellar keratoplasty are important risk factors for the development of elevated intraocular pressure and glaucoma—both short- and long-term post-op.6,7 In a study population of patients with a history of PK or Descemet’s stripping endothelial automated keratoplasty, one-third of eyes were determined to require postoperative IOP lowering or escalation of IOP-lowering therapy.6 Possible explanations for the OAG or worsening of disease other than elevated IOP due to steroid response include synechial angle closure, increased presence of pro-inflammatory mediators and intraoperative mechanical alteration of the angle that result in reduced trabecular outflow.6,7
History of glaucoma therapy and history of glaucoma surgery in addition to medical therapy are also important risk factors for corneal graft failure.6,8
Medication Considerations
The cytotoxic effects of benzalkonium chloride (BAK) on corneal epithelial cells and conjunctival goblet cells, and its effect of increasing inflammatory markers and tear film destabilization, have been well-described, but its impact on corneal endothelial cells in a clinical environment has not been established.9 In a laboratory setting, human corneal endothelial cells were determined to be less viable following exposure to BAK, which was more pronounced at higher concentrations over longer periods of time.10 The authors concluded that, due to the effective low-concentration of BAK exposure to corneal endothelial cells in comparison to that of corneal and conjunctival epithelial cells following topical ophthalmic medication instillation, the risk of corneal endothelial cell damage from BAK-preserved IOP-lowering medication was rare.10 However, considering the host of cytotoxic effects of BAK and the long-term exposure to ocular tissues through chronic therapy in glaucoma management, minimizing BAK load through preservative-free formulations is a reasonable approach to the extent that cost, coverage and access allow.
Of all IOP-lowering medications, dorzolamide and netarsudil are two agents that have been described to have potential impact on the corneal endothelium when topically applied. Dorzolamide reduces aqueous production through selective and temporary inhibition of carbonic anhydrase isozyme II present within the ciliary processes. The enzyme is also present within corneal endothelial cells and its inhibition may impact endothelial cell pump function in moderating stromal hydration.11 While not a contraindication, use topical ophthalmic carbonic anhydrase inhibitors in individuals with low corneal endothelial cell count with caution.12
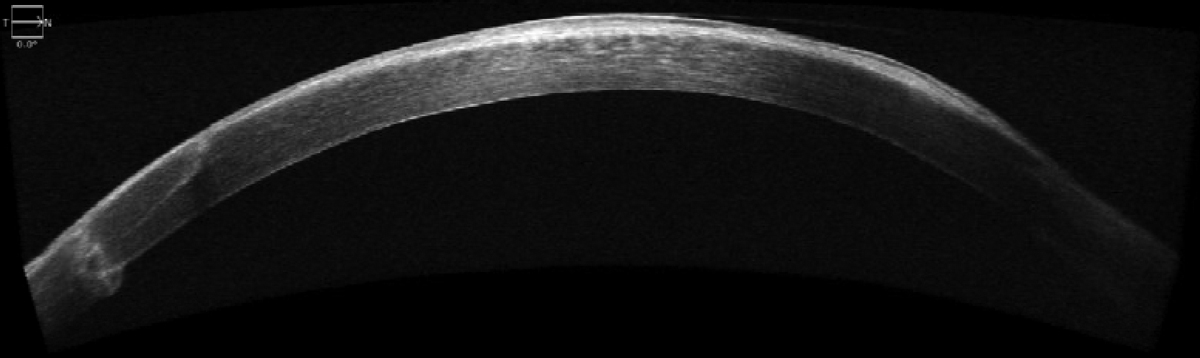 |
|
SD-OCT of the cornea following penetrating keratoplasty. Click image to enlarge. |
Corneal decompensation has been reported during or following treatment with dorzolamide in nine eyes with previous intraocular surgery and endothelial disease.13 Upon further review, each of the nine reported cases had multiple risk factors identified for potential causation of corneal edema and decompensation through mechanisms other than topical dorzolamide use.14 In individuals with ocular hypertension or OAG without endothelial abnormality, no significant difference in change in central endothelial cell density or corneal thickness was described in patients treated with dorzolamide in comparison to those treated with timolol or betaxolol.11 In patients with corneal guttata, increased central corneal thickness without decompensation has been measured following three times daily administration of topical dorzolamide for four weeks, supporting the recommendation to monitor patients who use topical dorzolamide in the context of pre-existing corneal endothelial disease.15
Netarsudil, a commercially available ROCK inhibitor compound, acts to lower IOP through increased trabecular meshwork outflow. In addition to its IOP-lowering effect, netarsudil has also been demonstrated to increase corneal endothelial cell proliferation in vitro and has been described as a topically administered adjunct in individuals who undergo Descemet stripping only in the management of Fuchs’.16 The use of topical netarsudil in patients with Fuchs’ endothelial dystrophy continues to be evaluated with a recent Phase II clinical trial identifying decrease in central corneal thickness with once and twice daily dosing with improved visual acuity and symptoms of glare.17
Takeaways
Considering the advanced optic neuropathy and associated functional vision loss, IOP-lowering therapy was escalated in the 68-year-old man’s case. He is currently treated with latanoprost 0.005% QHS OU in addition to dorzolamide-timolol fixed combination BID in the right eye and timolol 0.5% BID in the left eye. The post-PK patient has recently begun latanoprost 0.005% QHS OU with the goal of improved adherence and acceptable tolerability in comparison to previous therapies and is scheduled for follow-up in one month to assess medication efficacy and tolerability.
The common connection of OAG in the context of low endothelial cell count due to corneal transplant, or low endothelial cell count as a consequence of glaucoma surgery, requires long-term management of both optic nerve and corneal health to maximize visual outcome.
Dr. Steen is an associate professor at Nova Southeastern University College of Optometry where she serves as director of the Glaucoma Service, coordinator of the Primary Care with Emphasis in Ocular Disease Residency and teaches courses in glaucoma and ocular pharmacology. Her financial disclosures include Bausch & Lomb, Santen, Ocuphire and Carl Zeiss Meditec.
1. Rosenfeld C, Price MO, Lai X, et al. Distinctive and pervasive alterations in aqueous humor protein composition following different types of glaucoma surgery. Mol Vis. 2015;21:911-8. 2. Christakis PG, Kalenak JW, Tsai JC, et al. The Ahmed vs. Baerveldt Study: five-year treatment outcomes. Ophthalmology. 2016;123(10):2093-102. 3. Fang CEH, Mathew RG, Khaw PT, et al. Corneal endothelial cell density loss after glaucoma surgery alone or in combination with cataract surgery: a systematic review and meta-analysis. Ophthalmology. 2022;129(8):841-55. 4. Lass JH, Benetz BA, He J, et al. Corneal endothelial cell loss and morphometric changes five years after phacoemulsification with or without CyPass micro-stent. Am J Ophthalmol. 2019;208:211-8. 5. Francis BA, Hsieh A, Lai MY, et al.; Los Angeles Latino Eye Study Group. Effects of corneal thickness, corneal curvature, and intraocular pressure level on Goldmann applanation tonometry and dynamic contour tonometry. Ophthalmology. 2007;114(1):20-6. 6. Ward MS, Goins KM, Greiner MA, et al. Graft survival vs. glaucoma treatment after penetrating or Descemet stripping automated endothelial keratoplasty. Cornea. 2014;33(8):785e9. 7. Shree N, Gandhi M, Dave A, et al. Incidence and risk factors for post-penetrating keratoplasty glaucoma. Indian J Ophthalmol. 2022;70(4):1239-1245. 8. Writing Committee for the Cornea Donor Study Research Group; Sugar A, Gal RL, Kollman C, et al.. Factors associated with corneal graft survival in the Cornea Donor Study. JAMA Ophthalmol. 2015;133(3):246-54. 9. Goldstein MH, Silva FQ, Blender N, et al. Ocular benzalkonium chloride exposure: problems and solutions. Eye (Lond). 2022;36(2):361-8. 10. Ayaki M, Iwasawa A, Inoue Y. Toxicity of antiglaucoma drugs with and without benzalkonium chloride to cultured human corneal endothelial cells. Clin Ophthalmol. 2010;4:1217-22. 11. Lass JH, Khosrof SA, Laurence JK, et al. A double-masked, randomized, one-year study comparing the corneal effects of dorzolamide, timolol and betaxolol. Dorzolamide Corneal Effects Study Group. Arch Ophthalmol. 1998;116(8):1003-10. 12. Merck. Highlights of prescribing information: Trusopt (dorzolamide hydrochloride ophthalmic solution) 2%. www.accessdata.fda.gov/drugsatfda_docs/label/2014/020408s050lbl.pdf. Accessed September 6, 2023. 13. Konowal A, Morrison JC, Brown SV, et al. Irreversible corneal decompensation in patients treated with topical dorzolamide. Am J Ophthalmol. 1999;127(4):403-6. 14. Adamsons I. Irreversible corneal decompensation in patients treated with topical dorzolamide. Am J Ophthalmol. 1999;128(6):774-6. 15. Wirtitsch MG, Findl O, Heinzl H, et al. Effect of dorzolamide hydrochloride on central corneal thickness in humans with cornea guttata. Arch Ophthalmol. 2007;125(10):1345-50. 16. Din N, Cohen E, Popovic M, et al. Surgical management of fuchs endothelial corneal dystrophy: a treatment algorithm and individual patient meta-analysis of descemet stripping only. Cornea. 2022;41(9):1188-95. 17. Lindstrom RL, Lewis AE, Holland EJ, et al. Phase II, randomized, open-label parallel-group study of two dosing regimens of netarsudil for the treatment of corneal edema due to fuchs corneal dystrophy. J Ocul Pharmacol Ther. 2022;38(10):657-63. |

