 |
An 88-year-old Caucasian female presented to the clinic with acute onset of pain and swelling around her left eye. She stated that about a week prior she had an upper respiratory infection, and after blowing her nose, she noted her left eye had become red and swollen. She also had a headache localized behind the left eye that persisted after the illness passed. She mentioned the onset of a “whooshing” sound in her left ear and double vision that gradually worsened in intensity over the past week. Her extensive medical history included hypertension, high cholesterol, congestive heart failure, chronic obstructive pulmonary disease, deep vein thrombosis, hypothyroidism, anxiety/depression and a history of lung cancer about 30 years ago. Her current medications included Diovan (valsartan, Novartis), bumetanide, Lasix (furosemide, Sanofi-aventis), Eliquis (apixaban, Pfizer), Pravachol (pravastatin, Bristol-Myers Squibb), levothyroxine, albuterol and alprazolam.
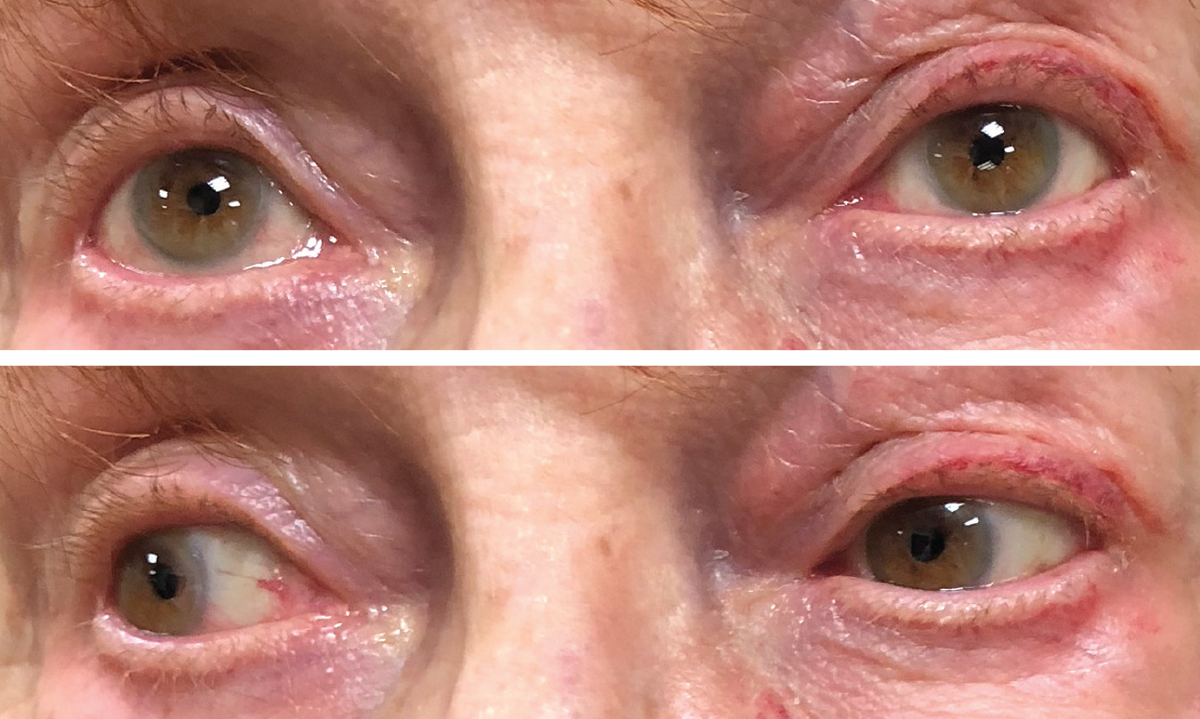 |
| Figs. 1 and 2. Upon initial exam, the patient showed upgaze restriction, above, and adduction deficit when looking to the right, below. Periorbital fluid and hemorrhage are noted in both images as well. Click images to enlarge. |
The Stats
On examination her best-corrected visual acuity (BCVA) was 20/30 in the right and left eyes. Intraocular pressures were 12mm Hg in the right eye and 14mm Hg in the left eye. Extraocular motilities were full on the right but showed upgaze and adduction restriction on the left (Figures 1 and 2). Cranial nerves three, four and six were intact. Exophthalmometry was 12mm OD and 16mm OS. Her pupils were normal and equal with no afferent pupillary defect. There was periorbital fluid and some hemorrhage around the left eye and some ptosis on the left eyelid. She had had cataract surgery OU, and her IOLs were in good position. Her retinal exam was normal except for minimal epiretinal membranes and posterior vitreous detachments in both eyes, which were noted in her past history and were stable.
Imaging ASAP
Given her symptoms and exam findings, my main concern was an orbital mass or process behind the left eye. Because she was taking Eliquis and the concern for any risk of orbital or intracranial hemorrhage, I decided to admit her to the hospital rather than send for outpatient testing. Prompt computed tomography (CT) of the orbits and CT-angiography (CTA) of the head and neck showed an enlarged left superior orbital vein with contrast filling on arterial phase imaging, which was highly suspicious for a left carotid cavernous fistula (CCF) (Figure 3).
I consulted neurology and neurosurgery, and they scheduled the patient for a diagnostic cerebral angiography to confirm the CCF with probable treatment the following day. Her Eliquis was stopped and she was switched to an aspirin and Plavix (clopidogrel, Bristol-Myers Squibb). The cerebral angiography showed a subtype A direct, high-flow CCF, which is high risk for intracranial hemorrhage.
Aftermath
Treatment was indicated, and a transarterial embolization was successful, with near complete resolution of the fistula.
The patient remained in the hospital for a number of days due to dyspnea, anxiety and decreased renal function; once these issues were managed and resolved, the patient was discharged. One month later upon re-examination, I noted all of her symptoms, including the proptosis and extraocular motility restriction, had resolved.
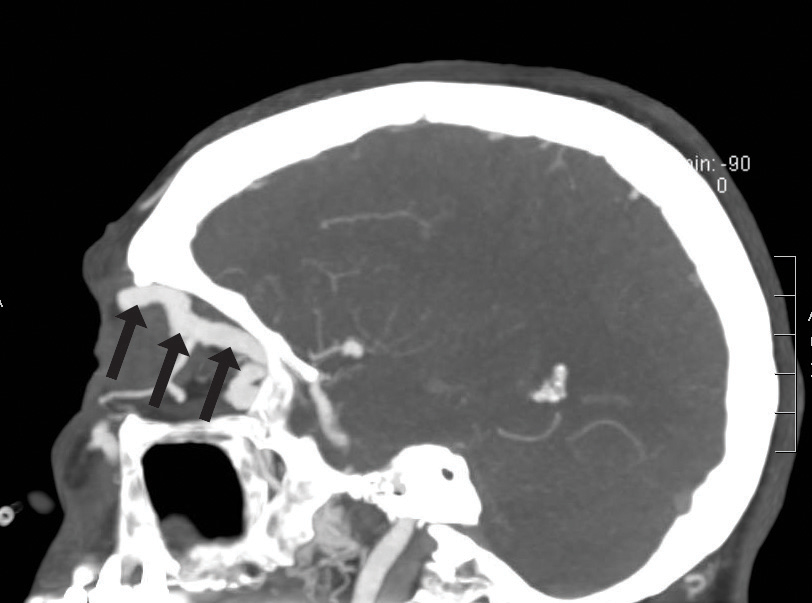 |
| Fig. 3. The patient’s CTA imaging demonstrates an enlarged left superior ophthalmic vein. |
Discussion
CCFs are abnormal connections between the carotid artery and the cavernous sinus.1 Various classifications of CCFs exist, based on etiology (traumatic or spontaneous), hemodynamics (high-flow or low-flow lesions) or anatomy or angiographically (direct when the fistula originates directly in the internal carotid artery, or indirect when the fistula originates in the dural branches of the carotid artery).1,2 Further characterization of CCFs are divided into four subtypes according to the arterial supply: subtype A is a direct, high-flow CCF between the internal carotid artery (ICA) and cavernous sinus; subtypes B, C and D are indirect, low-flow CCFs where the fistula originates in the dural branches of the carotid artery.1
The classification system is important from a neurosurgical standpoint because the treatment is specific for each entity.2
Ocular symptoms and clinical findings vary. Headache, retro-orbital pain, decreased vision and diplopia are typical patient complaints.
A direct CCF, as seen in this patient, is a high-flow lesion where blood flows from a high-pressure compartment (the ICA) to a low-pressure compartment (the cavernous sinus).1 When this occurs, it causes an increase in pressure in the cavernous sinus, which can result in cranial nerve three, four, five and six palsies.1,2 In this patient’s case, her motility restriction was not due to a nerve palsy, but to the enlarged ophthalmic vein.
Additionally, this increase in pressure leads to engorgement of the ophthalmic veins, causing symptoms such as ocular bruit, chemosis and pulsatile proptosis.1 Direct, high-flow CCFs are most commonly a result of trauma. When spontaneous or outside the setting of trauma, they are generally associated with a ruptured intracavernous aneurysm, Ehlers-Danlos syndrome or fibromuscular dysplasia.1,2 In this patient’s case, she denied any prior trauma and did not have any risk factors for this direct CCF. While blowing her nose likely did not cause the CCF, it is possible the pressure it created exacerbated the pre-existing condition.
Indirect, low-flow CCFs do not pose a significant risk for hemorrhage and can be monitored. If the patient cannot tolerate the symptoms or if there are ocular morbidities stemming from a low-flow fistula, endovascular treatment is recommended.
Because direct, high-flow CCFs have an 8.4% risk of hemorrhage, treatment is always recommended.3 The main goal for treatment is exclusion of the fistula while maintaining patency of the ICA.1 This is typically achieved with endovascular embolization with a combination of detachable balloons, coils, stents or liquid embolic agents.4
Once the diagnosis has been made, the OD’s role is to continue to monitor all findings that were present on initial exam and to follow for resolution after neurosurgical intervention. Symptoms from a CCF will significantly improve following treatment. Resolution of symptoms and clinical findings can occur hours or days after treatment but can sometimes take weeks to months.1
1. de Aguiar GB, Jory M, Silva JM, et al. Advances in the endovascular treatment of direct carotid-cavernous fistulas. Rev Assos Med Bras. 2016; 62(1):78-84. |

