An 80-year-old white male presented for a postoperative follow-up examination. A cataract was removed from his left eye one month earlier. He reported decreasing vision in his right eye, which was secondary to a nasal pterygium encroaching the visual axis. The growth had been there for several years. Upon questioning, the patient reported that he would like the pterygium removed.
The patients ocular history included primary open-angle glaucoma and pseudophakia in both eyes. His medications were Xalatan (latanoprost, Pfizer), one drop in both eyes at bedtime, and Refresh LiquiGel (Allergan) as needed for ocular irritation. He reported no known drug allergies.
Diagnostic Data
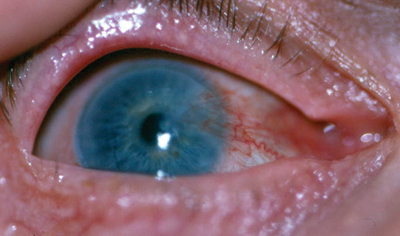
The patients best-corrected visual acuity was 20/50-2 O.D. Slit lamp examination revealed a nasal pterygium extending 6mm onto the cornea in his right eye (figure 1).
 |
|
1. This patients pterygium extended 6mm onto the cornea of his right eye. |
We diagnosed the patient with a stage-3 pterygium on his right eye (see Pterygia Grading Scale, below).
| Pterygia Grading Scale7 |
| Stage | Growth onto Cornea |
| Stage 1 |
0mm to 2mm |
| Stage 2 | 2mm to 4mm |
| Stage 3 | >4mm |
Treatment and Follow-Up
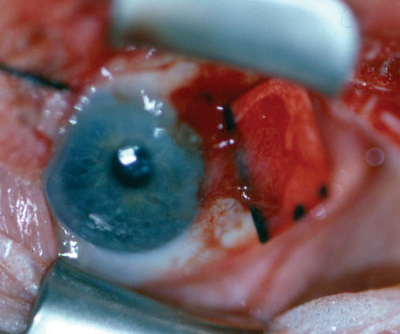
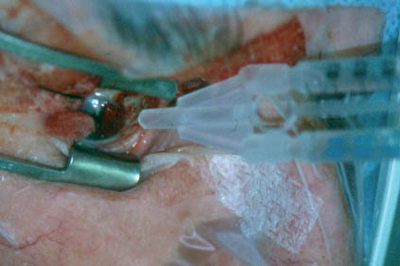
We performed a pterygium excision and conjunctival autograft transplantation. This technique involves transferring a free autologous superior bulbar conjunctival graft using a rigid paper template. The graft covers the bare sclera exposed by pterygium excision (figure 2).1,2 We used fibrin sealant to adhere the graft to the sclera (figure 3). Fibrin sealants stop bleeding and aid in adhesion in surgical procedures.
 |
|
2. The graft settles on the rigid template as it is positioned over bare sclera. |
 |
|
3. Tisseel fibrin sealant is applied to bare sclera. |
 |
|
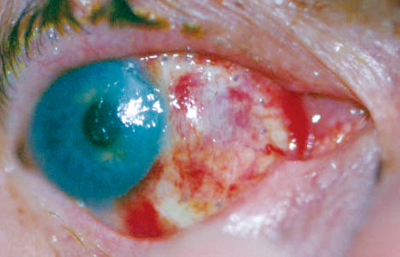
4. At one-day postoperatively, the graft was well-positioned, and there did not appear to be an underlying hematoma. |
We saw the patient again two weeks after surgery. He reported little scratchiness and good vision. The graft was re-vascularized and viable. The sutures were still in place. We discontinued TobraDex and prescribed Pred Forte 1% (prednisolone acetate, Allergan) four times a day for two weeks.
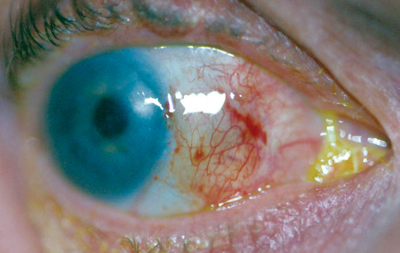
At the one-month follow-up examination, the graft was viable and well-approximated (figure 5). The patient did not report any problems.
 |
|
5. At one-month postoperatively, the graft was viable and well-approximated, and the patient did not report any problems. |
Discussion
Pterygia are histopathologic changes that occur in normal conjunctival tissue. They are characterized by elastodysplasia and elastodystrophy of subconjunctival connective tissue.4 Pterygia are classified as primary or recurrent and are graded according to stage.
The primary reasons that people undergo pterygium excision are visual axis involvement and astigmatism that reduces visual acuity. Extensive pterygia can cause subconjunctival fibrosis that can limit ocular motility, which in turn may lead to diplopia. Patients may also experience contact lens intolerance, chronic irritation and cosmetic deformity.5
The primary goal of pterygium removal is to prevent recurrence. Some removal techniques include:
Bare sclera. In this technique, the surgeon removes the pterygium and sutures the remaining healthy conjunctiva to the sclera, leaving the scleral area of removal exposed. This method is seldom used because a bare sclera invites ocular infection secondary to postoperative contamination.3 This method also has the highest rate of recurrence.6
Simple closure or sliding closure. This technique is a fast and easy method of excision. The surgeon dissects the pterygium and closes the remaining conjunctiva with interrupted sutures at the wound edges with little or no sclera exposed.3,7 This technique and bare sclera are reserved for small pterygia.
Rotational and free autologous conjunctival autografts. These can be used for most pterygium surgeries, especially those that require a large area of resection. The surgeon can rotate the conjunctival graft over the excision site or take a graft from another area of the conjunctiva. We decided to use the latter technique with our patient. The surgeon then sutures the graft into place.3,7
In the standard graft procedure, surgeons harvest the graft from the supero-temporal quadrant of conjunctiva. Some surgeons prefer to use the inferior conjunctiva as a donor site to preserve the superior aspect for possible future glaucoma filtration surgery.4,8 A similar technique for pterygium excision engages the use of an amniotic membrane to cover the resection bed in a similar manner to the autologous conjunctival autograft. The drawback to using an amniotic membrane is its cost.7
An adjunctive therapy to the bare sclera and simple closure techniques is mitomycin C. This antimitotic agent prevents proliferation of fibroblastic cells; it can be used intraoperatively and postoperatively.7 This therapy also can reduce the pterygium recurrence rate. However, complications can include glaucoma, severe pain, scleral necrosis, dellen formation and scleral perforation.3,5,6
Other postoperative adjunctive therapies include beta irradiation and thiotepa. Both are antimitotics that can be used in conjunction with bare sclera. However, they are rarely used today due to adverse complications, such as scleral perforation, sclerokeratitis and inflammation.7
|
Recurrence Rates of Pterygia |
| Treatment | Recurrence |
| Conjunctival autograft1,2,4,6,11 |
0% to 40% |
| Mitomycin C5,6 |
6% to 10% |
| Bare sclera6 |
Up to 80% |
| Simple closure6 |
45% to 70% |
| Sliding conjunctival flap6 |
1% to 5% |
To reduce complications, surgeons may use a fibrin sealant in pterygium surgery. Tisseel fibrin sealant (Baxter Healthcare Corporation), the first fibrin sealant to be approved by the FDA, is a combination of a highly concentrated solution of fibrinogen and thrombin. Fibrinogen is a protein from human blood that forms a clot when combined with thrombin. Thrombin is a protein that facilitates blood clotting. The combination produces a natural substance that replicates the blood clotting cascade process to stop bleeding and aid in adhesion in surgical procedures.9
There are several advantages to using fibrin sealants. First, they cause rapid tissue adhesion and decrease the number of sutures required. This speeds up the healing process and decreases the discomfort caused by sutures. The sealant is naturally bioabsorbable as it dissolves and exhibits elasticity.9 A greater continuous surface area is possible with fibrin sealants than with traditional sutures. This is because the sealant does not allow blood and serous fluid to collect between the conjunctiva and the scleral bed, which can cause the graft to tent up, resulting in a dellen.8
A recent study showed that the antibiotics cefotaxime, mezlocillin, gentamicin, neomycin and polymyxin B can decrease the rate of clot formation or the strength of the clot when mixed with the fibrin sealant.10 Much of the antibiotic is released from the fibrin clot within 24 hours, although it may remain in the tissue for longer periods. We did not use an antibiotic in the sealant for this patient.
The addition of a fibrin sealant during pterygium surgery can reduce the number of sutures needed in autologous conjunctival grafts, increase patient comfort and decrease recovery time. Fibrin sealant can improve hemostasis, reduce blood loss and cause tighter tissue adhesion for faster healing. The sealant is a promising new approach that may decrease the incidence of postoperative infections and the rate of pterygia recurrence.
Dr. Mittleider-Heil is in private practice in Bismark, N.D., and Dr. Skorin is a staff ophthalmologist at the Albert Lea Eye Clinic, Mayo Health System, Albert Lea, Minn.
1. Kenyon KR, Wagoner MD, Hettinger ME. Conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology 1985 Nov;92(11):1461-70.
2. Shaw EL. A modified technique for conjunctival transplant. CLAO J 1992 Apr;18(2):112-6.
3. Gans LA. Surgical treatment of pterygium. In: Belin MW, ed. Focal points. Clinical modules for ophthalmologists. San Francisco: American Academy of Ophthalmology, 1996:14;1-9.
4. Syam PP, Eleftheriadis H, Liu CSC. Inferior conjunctival autograft for primary pterygia. Ophthalmology 2003 Apr;110(4):806-10.
5. Donnenfeld ED, Perry HD, Fromer S, et al. Subconjunctival mitomycin C as adjunctive therapy before pterygium excision. Ophthalmology 2003 May;110(5):1012-26.
6. Hirst LW. The treatment of pterygium. Surv Ophthalmol 2003 Mar-Apr;48(2):145-77.
7. Donaldson KE, Alfanso EC. Recent advances in pterygium surgery. Contemporary Ophthalmology 2003;25:1-7.
8. Safran SG. Fibrin glue replaces sutures in pterygium surgery. Ocular Surgery News 2004;3:45-6.
9. Stoebner B. Glue for eye surgery. Pacific Cataract and Laser Institute Newsletter 2004; Winter. www.pcli.com/od/newsletter/2004/4/. (Accessed April 12, 2006).
10. Thompson DF, Davis TW. The addition of antibiotics to fibrin glue. South Med J 1997 Jul;90(7):681-4.
11. Fayez MFA. Limbal versus conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology 2002 Sep;109(9):1752-5.

