
A mothers body undergoes many changes throughout the course of a pregnancy. Every major organ system is affectedincluding the eyes. The most common ocular changes involve intraocular pressure, the cornea and visual fields, but retinal changes can also be present and deserve the attention of the attending optometrist.
The conditions of pregnancy that affect the retina may be broken into two categories: preexisting conditions worsened by pregnancy, and pathologic changes caused by pregnancy.
Preexisting Conditions
Diabetic retinopathy. Pregnancy influences the natural progression of diabetic retinopathy (DR). The exact mechanism is unknown, but DR may worsen during pregnancy and regress postpartum.1 So, a comprehensive examination with regularly scheduled follow-up is essential.2-8
Diabetic changes that occur during pregnancy are similar to those that occur in non-pregnant patients, and there is no difference in grading (figure 1).
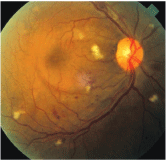
1. A patient with moderate NPDR should be monitored every four to six weeks with a dilated examination.
Several risk factors for the progression of retinopathy in pregnancy have been documented in the literature. Metabolic control, duration of diabetes, baseline severity of retinopathy, retinal blood flow and hypertension must be assessed and followed closely throughout the pregnancy (see Risk Factors for the Progression of Diabetic Retinopathy).2-6 But, gestational diabetes (diabetes mellitus that occurs during pregnancy) has not been associated with the development of retinopathy.5
Risk Factors for the Progression of Diabetic Retinopathy: Concurrent hypertension. Poor metabolic control. Longer duration of diabetes. Increased severity of baseline retinopathy. Impaired retinal blood flow.
Several studies outline the rates of progression for DR. Patients with minimal to no DR at the onset of pregnancy showed progression at an 8% rate, while women who had proliferative disease at the onset of pregnancy progressed at a rate of 25%.1 A second study shows an even greater disparity: no progression in patients with little to no retinopathy vs. progression of 65% in those with frank retinopathy.9
The prospective Diabetes in Early Pregnancy Study (DEPS) followed 155 women with diabetes from preconception to one month postpartum. In the 140 patients with a clear fundus at baseline, progression was seen in 10.3% of patients with no retinopathy, 21.1% of patients with microaneurysms, 18.8% of patients with mild nonproliferative diabetic retinopathy (NPDR) and 4.8% of patients with moderate-to-severe NPDR at baseline. Proliferative diabetic retinopathy developed in 6.3% with mild, and 29% with moderate-to-severe baseline retinopathy.10 Another study found that 81% of patients continued to progress to proliferative DR within two months postpartum.1 In these cases, laser photocoagulation was required.
Treating diabetic retinopathy in a pregnant woman is no different than treating the standard patient, although follow-up must be condensed (see Recommendations for Monitoring Pregnant Patients with Diabetes). In patients without DR or microaneurysms, a dilated exam should be performed in the first trimester and repeated as needed based on visual complaints.
Recommendations for Monitoring Pregnant Patients with Diabetes Condition Follow up as needed for visual complaints. Fundus photography in first trimester and with progression. Dilated exam every four to six weeks. Fundus photography in first trimester and with progression. Dilated exam every four to six weeks, or more frequently as needed. Laser photocoagulation, if severe. Proliferative diabetic retinopathy. Fundus photography in first trimester and with progression. Dilated exam every two weeks, or more frequently as needed. Laser photocoagulation.
Recommended Management
No diabetic retinopathy.
Dilated eye exam in first trimester.
Follow up as needed for visual complaints.
Microaneurysms only.
Dilated eye exam in first trimester.
Mild to moderate diabetic retinopathy.
Dilated eye exam in first trimester.
Preproliferative diabetic retinopathy.
Dilated eye exam in first trimester.
Dilated eye exam in first trimester.
For mild to moderate NPDR, a dilated exam should be performed every four to six weeksmore frequently if visual complaints are present. Laser photocoagulation should be considered based on severity.11 If proliferative changes are noted, photocoagulation should be initiated on a restricted or limited basis.
Postpartum regression occurs in most cases, and its rate and timing are currently under investigation.1 The best course of action is to treat all proliferative changes with photocoagulation prior to pregnancy, if possible.2-6
Macular edema may also occur during the course of the pregnancy with or without proliferative retinopathy. In some patients, the edema regresses postpartum, while in others, it persists, causing long-term visual loss.12 Laser photocoagulation may be used to treat the edema, but it may also exacerbate the condition. Salt-restriction diets and diuretics have been utilized with only minimal success. Monitor patients for up to one year after the pregnancy to prevent any possible vision-threatening complications.11
Sarcoidosis. This systemic granulomatous disease of unknown etiology frequently affects women during their reproductive years.13,14 Sarcoidosis can affect women during pregnancy or occur up to one year postpartum. The literature is unclear regarding the effect of pregnancy on the course of the disease. Several recent studies indicate that pregnancy has a favorable effect, but others are not as concrete.13
Patients with normal chest X-rays prior to conception will likely remain normal. If resolution has already begun at the time of conception, it should continue during pregnancy. Those with inactive disease but residual changes as seen by X-ray will remain stable; but, those with active disease will show partial or total resolution during pregnancy.13
There are no documented cases of inactive sarcoid patients experiencing reactivation as a result of pregnancy. No increased frequency of miscarriage, obstetric complications or congenital abnormalities have been linked to those suffering from the disease.13
Toxoplasmosis. The parasite Toxoplasma gondii can be acquired congenitally via acute maternal infection during pregnancy or ingestion of infected meat.15-18 Infection is asymptomatic in most patients, but in 10% of cases, it causes a self-limiting, non-specific illness that rarely requires treatment.17 During pregnancy, recurrence of toxoplasmic retinochoroiditis is more common than a new diagnosis (figure 2).
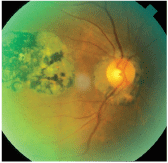
2. In an inactive case of toxoplasmosis, transmission to the fetus will not occur.
Immune system suppression predisposes a pregnant patient to recurrence of ocular toxoplasmosis, which may present an opportunity for a more aggressive form of the disease to develop.19 The severity of the congenital infection is highest when acquired during the first trimester; yet, the frequency of transmission is greatest during the third trimester. Due to maternal immunity, women with reactivation of latent toxoplasmosis or scars do not transmit this disease to the fetus.20
While the majority of physicians in one survey indicated that they would treat only those pregnant women with severe, vision-threatening acute toxoplasmosis, options are limited.21 Due to the teratogenic nature of pyrimethamine and the side effects of sulfonamides, the treatments of choice are the macrolides (spiramycin, azithromycin and clindamycin).19 One study found good visual outcomes when intraocular clindamycin and dexamethasone was used concurrently with systemic sulfadiazine.22
Pathologic Changes
Central serous chorioretinopathy (CSCR). This disorder is characterized by a localized serous retinal detachment of the neurosensory retina in the macula. It most commonly affects adults between the ages of 20 and 45. CSCR affects men more often than women, at a ratio of 10 to one.23-26 Pregnancy is considered a risk factor for CSCR development in women.24 For example, a recent study found that women who are or were pregnant are 7.1 times more likely to develop CSCR than an age-matched group with no history of pregnancy.11
Hormonal and hemodynamic changes, ischemia, and permeability changes in vasculature have been proposed to explain the development of CSCR, but none have been proven to be a definitive cause.26
CSCR can occur at any time during pregnancy. Subjective complaints include blurred vision, metamorphopsia, color changes and darkening of the central visual field (figure 3).23 Recurrence in the same eye in successive pregnancies has been documented.27 CSCR associated with pregnancy, compared with cases in men and non-pregnant women, has been found to cause increased amounts of subretinal fibrous exudates.11
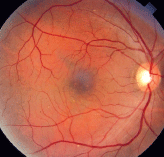
3. CSCR typically resolves within weeks to months, so treatment is supportive.
Treatment, as with traditional CSCR, is supportive. Focal laser photocoagulation can be delayed, since this condition often resolves within one or two months postpartum.11
Pseudotumor cerebri (PTC). PTC is a syndrome of increased intracranial pressure without hydrocephalus; it is seen in obese women and women of childbearing age.28 Recent studies have debunked earlier beliefs that PTC is found in a higher percentage of pregnant women vs. the general population.28-30 PTC is not considered a contraindication to pregnancy, and most patients can be managed with minimal care.31
Most pregnant patients with PTC develop symptoms in the first trimester, although it may be diagnosed throughout pregnancy (figure 4). And, some women relapse during subsequent pregnancies.31 Common symptoms include headaches, vomiting, tinnitus, diplopia and visual disturbances, such as transient visual obscurations, visual field loss (enlarged blind spot) or loss of central visual acuity.31,32
4. Pseudotumor cerebri is not a contraindication to pregnancy, and it can be managed with minimal care.
Once diagnosed, the decision to treat is based on visual acuity and visual field loss. Monitoring, including visual field testing, should occur every two to three months in patients with no visual loss.30 Optic atrophy and permanent visual field loss may occur following resolution, but pregnancy does not affect the eventual visual outcome of patients.15,32,33
Lumbar puncture is considered risky due to the chance of spontaneous miscarriage. Steroids are used by many obstetricians, even though there have been documented side effects in both mother and fetus.34
Dehydrating agents, including carbonic anhydrase inhibitors, loop diuretics and mannitol, are contraindicated due to fetal risk.35 Acetazolamide can be used after 20 weeks if necessary. Optic nerve sheath decompression and lumboperitoneal shunting are acceptable treatments during pregnancy to prevent significant visual compromise.31 Decompression requires less time under anesthesia (a worthwhile consideration in this patient base).
Hypertensive disorders. Pregnancy-induced hypertension (PIH) can be classified as either preeclampsia or eclampsia. Preeclampsia includes hypertension, edema and proteinuria. Eclampsia includes this triad and also accounts for the incidence of convulsions.1 Pregnancy-aggravated hypertension, which is either preeclampsia or eclampsia in conjunction with chronic hypertension, is another category.36
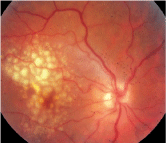
The visual system is affected in 30% to 100% of patients with preeclampsia.37 Complaints include blurred vision, photopsia, loss of vision (central or total) and diplopia.15,38-40 Ophthalmoscopic findings include reduced arteriole-to-venule ratio, retinal hemorrhage, edema, cotton-wool spots, serous exudative retinal detachment and choroidal infarcts (figure 5).41 The severity of both preeclampsia and fetal mortality has been associated with corresponding retinal arteriolar narrowing.40 Localized or generalized constriction occurs in nearly 60% of patients with preeclampsia.11
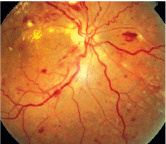
5. Retinal findings in hypertensive retinopathy.
Serous exudative detachments have occurred in both preeclampsia and eclampsia at a rate of 1% to 2% or 10%, respectively.42,43 Though the exact mechanism is not known, they are usually observed in the absence of significant retinal vascular abnormalities. Resolution typically occurs within a few weeks postpartum.15 In one study, 72% of cases resolved within the first week, and only one out of 40 persisted beyond three weeks. Long-term visual changes may occur due to retinal pigment epithelium changes or optic atrophy.42,44,45
Disseminated intravascular coagulation (DIC). This complex coagulation disorder occurs when blood clots form in small blood vessels throughout the body.46-49 While the renal cortex, heart and brain are most commonly involved, the eye can also be affected. DIC occurs as a secondary complication to an underlying systemic condition.47 It is most commonly associated with abruptio placentae (premature separation of the placenta), intrauterine death, acute leukemia and severe burns, but it may also occur with severe preeclampsia, septic- or saline-induced abortion, systemic infections (especially gram-negative) and drug reactions.46-48 Although it may sometimes develop rapidly, cases of insidious onset with minimal signs and symptoms have been reported.47
The choroid is the most common ocular location for DIC to manifest. Patients often complain of visual loss from choroidal infarction, choroidal hemorrhage, retinal pigment epithelial (RPE) detachment or serous posterior pole detachments. Vessels of the iris, ciliary body, retina, sclera and posterior ciliary circulation are not usually affected. Hemorrhage into the subdural space surrounding the optic nerve, subretinal space, retina subhyaloid space, vitreous body or anterior chamber has been documented in some patients.47
Visual recovery usually occurs once the DIC resolves. Occluded vessels are recanalized, and RPE function gradually improves. Mild pigmentary changes and decreased vision may persist, and visual improvement may take time.46
Thrombotic thrombocytopenia purpura (TTP). This rare disorder is characterized by small vessel thrombosis, thrombocytopenia, microangiopathic hemolytic anemia and neurologic and renal dysfunction.50-52 It is more prevalent in females than males by a ratio of 3:2, and the median affected age is 35. Several precipitating factors have been linked with TTP, including pregnancy and postpartum complications, viral and bacterial infections, autoimmune disease (e.g., lupus or Sjgrens syndrome) and medications such as heparin, the penicillins, sulfonamides and oral contraceptives.50
One-third of published cases contain visual findings.51 Visual symptoms may occur due to serous retinal detachment, retinal artery narrowing, retinal hemorrhage and optic nerve head edema. The central nervous system may be involved, which leads to pupillary changes, ocular palsy, homonymous hemianopia and cortical blindness.50
TTP is typically treated with plasmapheresis. Corticosteroids (to reduce inflammation), as well as vincristine, cyclophosmamide, splenectomy and rituximab, a monoclonal antibody that targets B-cell lymphocytes, have been used in conjunction with traditional therapies. Ocular therapy is supportive. Fluorescein angiography should be performed at onset to establish baseline retinal and choroidal vessel functioning, as well as when documenting any changes that occur. Those patients who develop neovascularization are candidates for focal panretinal photocoagulation.50,52
Antiphospholipid syndrome (APLS). In this autoimmune disease, patients test positive for lupus anticoagulant and anticardiolipin antibody.53-56 Thrombosis, the main feature, may be either arterial or venous and may affect any size vessel in the body. APLS commonly causes recurrent miscarriage, transient ischemic attack, migraine, pulmonary and labile hypertension, epilepsy thrombocytopenia and ocular ischemia. While the epidemiological picture is not clear, APLS is found most often in patients with Sjgrens syndrome, rheumatoid arthritis, viral infection (e.g., HIV, adenovirus, rubella or mumps) and non-viral infections (e.g., syphilis, borreliosis or typhoid fever).55
Ocular ischemia and fundus findings are found in up to 88% of patients. Ophthalmic manifestations may present in the anterior and posterior segment, and may be neurological in nature. The anterior segment is typically not involved; but if present, symptoms are usually mild. Episcleritis, limbal and filamentary keratitis, and iritis are found. Posterior segment manifestations include vasculitis, retinal detachment and venous tortuosity.
Cases of branch retinal vein occlusion, branch retinal artery occlusion, central retinal vein occlusion and bilateral choroidal infarction have been documented in the literature, as well as findings of cotton-wool spots, retinal hemorrhages and central serous retinopathy.55
Neuro-ophthalmic findings are also common. Monocular/binocular transient vision or visual field loss, ischemic optic neuropathy and progressive optic nerve atrophy have been reported. Single or multiple cranial nerve palsies have been associated with APLS.53
Rhegmatogenous retinal detachment. In the past, risk of new retinal tears or detachments in patients with high myopia or previous retinal complications who underwent spontaneous vaginal delivery was cause for concern. Several studies have since put such concerns to rest, but eye-care providers and obstetricians continue to educate patients regarding the signs and symptoms of retinal detachment.57
Several studies have demonstrated no deleterious effects on the retina with spontaneous vaginal delivery. In each of three studies, patients had at least one risk factor for rhegmatogenous retinal detachment, though none occurred.58-60 Yet, in a survey performed in 2003, 64 obstetricians were questioned regarding risk of retinal detachment during labor. Almost half believed that a previous retinal detachment would put the patient at high risk, necessitating a caesarian section.57
6. A repaired retinal detachment is not a contraindication to a vaginal delivery.
While rhegmatogenous retinal detachment has not been reported in association with the act of childbirth, a few rare cases of detachment during pregnancy have been reported (figure 6).61 In such situations, treatment is controversial. The effect of labor and delivery on a detached or recently repaired retina is unknown. Little literature supports repair of the detachment either prior to delivery or immediately thereafter.
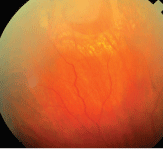
Courtesy: Mohammad Rafieetary, O.D., Charles Retina Institute
While many common systemic and ocular changes have been documented, there is still a risk for many other diseases to occur and/or become exacerbated during pregnancy. Some of the first steps you can take: Take a proper history, and educate your potentially pregnant patients. If the need for treatment should arise, consult with the patients obstetricians before prescribing. By taking extra care, treating and managing pregnant patients is a task that any optometrist will be able to accomplish.
Dr. Taub is an assistant professor in the pediatrics/binocular vision department at Southern College of Optometry. Dr. Lievens is an associate professor and chief of the primary care service at Southern College of Optometry.
1. Chan WC, Lim LT, Quinn MJ, et al. Management and outcome of sight-threatening diabetic retinopathy in pregnancy. Eye 2004 Aug;18(8):826-32.
2. Oguz H. Diabetic retinopathy in pregnancy: effects on the natural course. Semin Ophthalmol 1999 Dec;14(4):249-57.
3. Best RM, Chakravarthy U. Diabetic retinopathy in pregnancy. Br J Ophthalmol 1997; 81(3):249-51.
4. Sheth BP. Does pregnancy accelerate the rate of progression of diabetic retinopathy? Curr Diab Rep 2002 Aug;2(4):327-30.
5. Soubrane G, Coscas G. Influence of pregnancy on the evolution of diabetic retinopathy. Int Ophthalmol Clin 1998 Spring;38(2):187-94.
6. Aiello LP,
7. Moloney JB, Drury MI. The effect of pregnancy on the natural course of diabetic retinopathy. Am J Opthalmol 1982 Jun;93(6):745-56.
8. Klein R, Klein BE, Moss SE, et al. The
9. Laatikainen L, Larinkari J, Teramo K. Occurrence and prognostic significance of retinopathy in diabetic pregnancy. Metab Paediatr Ophthalmol 1980;4(4):191-5.
10. Chew EY, Mills JL, Metzger BE, et al. Metabolic control and progression of retinopathy. The Diabetes in Early Pregnancy Study. National
11. Schultz KL, Birnbaum AD, Goldstein DA. Ocular disease in pregnancy. Curr Opin Ophthalmol 2005 Oct;16(5):308-14.
12. Sinclair SH, Nesler C, Foxman B, et al. Macular edema and pregnancy in insulin-dependent diabetes. Am J Ophthalmol 1984 Feb;97(2):154-67.
13. Selroos O. Sarcoidosis and pregnancy: a review with results of a retrospective survey. J Intern Med 1990 Apr;227(4):221-4.
14. Haynes de Regt R. Sarcoidosis and pregnancy. Obstet Gynecol 1987 Sep;70(3 Pt 1):369-71.
15. Sunness JS. The pregnant womans eye. Surv Ophthalmol 1988 Jan-Feb;32(4):219-38.
16. Somani, S. Pregnancy, special considerations. www.emedicine.com/oph/topic747.htm (Accessed January 2005).
17. Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet 2004 Jun 12;363(9425):1965-76.
18. Brzin AP, Thulliez P, Couvreur J, et al. Ophthalmic outcomes after prenatal and postnatal treatment of congenital toxoplasmosis. Am J Ophthalmol 2003 Jun;135(6):779-84.
19. Kump LI, Androudi SN, Foster CS. Ocular toxoplasmosis in pregnancy. Clin Experiment Ophthalmol 2005 Oct;33(5):455-60.
20. Dunn D, Wallon M, Peyron F, et al. Mother-to-child transmission of toxoplasmosis: risk estimates for clinical counseling. Lancet 1999 May 29;353(9167):1829-33.
21.
22. Martinez CE, Zhang D,
23. Sunness JS, Haller JA, Fine SL. Central serous chorioretinopathy and pregnancy. Arch Ophthalmol 1993 Mar;111(3):360-4.
24. Haimovici R, Koh S, Gagnon DR, et al. Risk factors for central serous chorioretinopathy: a case-control study. Ophthalmology 2004 Feb;111(2):244-9.
25. Ko W. Central serous chorioretinopathy associated with pregnancy. J Ophthalmic Nurs Technol 1992 Sep-Oct; 11(5):203-5.
26. Gass JD. Central serous chorioretinopathy and white subretinal exudation during pregnancy. Arch Ophthalmol 1991 May;109(5):677-81.
27. Chumbley LC, Frank RN. Central serous retinopathy and pregnancy. Am J Ophthalmol 1974 Feb;77(2):158-60.
28. Digre KB, Varner MW, Corbett JJ. Pseudotumor cerebri and pregnancy. Neurology 1984 Jun;34(6):721-9.
29. Kassam SH, Hadi HA, Fadel HE, et al. Benign intracranial hypertension in pregnancy: current diagnostic and therapeutic approach. Obstet Gynecol Surv 1983 Jun;38(6):314-21.
30. Giuseffi V, Wall M, Siegel PZ, Rojas PB. Symptoms and disease associations in idiopathic intracranial hypertension (pseudotumor cerebri): a case-control study. Neurology 1991 Feb;41(2 Pt 1):239-44.
31. Evans RW, Friedman DI. Expert Opinion: the management of pseudotumor cerebri during pregnancy. Headache 2000 Jun;40(6):485-97.
32. Ellent R. Pseudotumor cerebri during pregnancy. Clin Eye Vision Care 1999 Jan;10(4):189-94.
33. Boddie HG, Banna M, Bradley WG. Benign intracranial hypertension. A survey of the clinical and radiological features and long-term prognosis. Brain 1974 Jun;97(2):313-26.
34. Swanson MW. Spontaneous regression of pregnancy associated papilledema. South J Optom 1991;9:26-31.
35. Corbett JJ, Thompson HS. The rational management of idiopathic intracranial hypertension. Arch Neurol 1989 Oct;46(10):1049-51.
36. Pritchard JA, MacDonald PC, Grant NF. Williams Obstetrics. 17th ed.
37. Sathish S, Arnold JJ. Bilateral choroidal ischaemia and serous retinal detachment in pre-eclampsia. Clin Experiment Ophthalmol 2000 Oct;28(5):387-90.
38. Dinn R, Harris A, Marcus PS. Ocular changes in pregnancy. Obstet Gynecol Surv 2003 Feb;58(2):137-44.
39. Dieckmann WJ. The Toxemia of Pregnancy. 2nd ed.
40. Jaffe G, Schatz H. Ocular manifestations of preeclampsia. Am J Ophthalmol 1987 Mar 15;103(3 Pt 1):309-15.
41. Capoor S, Goble RR, Wheatley T, Casswell AG. White-centered retinal hemorrhages as an early sign of preeclampsia. Am J Ophthalmol 1995 Jun;119(6):804-6.
42. Fry WE. Extensive bilateral retinal detachment in eclampsia with complete reattachment. Arch Ophthalmol 1929; 1;609-14.
43. Hallum AV. Eye changes in hypertensive toxemia of pregnancy. JAMA 1936;106:1649-1651.
44. Ballantyne AJ, Michaelson IC. Textbook of the Fundus of the Eye. 2nd ed. Baltimore, Williams and Wilkins, 1970:182-3.
45. Crowther WL, Hamilton JB. Eclampsia with amaurosis due to detachment of the retina. Med J Aust 1932;2:177-8.
46. Hoines J, Buettner H. Ocular complications of disseminated intravascular coagulation (DIC) in abruptio placentae. Retina 1989;9(2):105-9.
47. Samples JR, Buettner H. Ocular involvement in disseminated intravascular coagulation (DIC). Ophthalmology 1983 Aug;90(8):914-6.
48. Cogan DG. Ocular involvement in disseminated intravascular coagulopathy. Arch Ophthalmol 1975 Jan; 93(1):1-8.
49. Levi, M. Disseminated intravascular coagulation. Crit Care Med 2007 Sep; 35(9):2191-5.
50. Black RL, Terry JE. Ocular manifestations of thrombotic thrombocytopenic purpura. J Am Optom Assoc 1991 Jun; 62(6):457-61.
51. Jellie HG, Gonder JR, Canny CL, et al. Ocular involvement in thrombotic thrombocytopenic purpura: the angiographic and histopathological features. Can J Ophthalmol 1984 Oct;19(6):279-83.
52. Lambert SR, High KA, Cotlier E, Benz EJ Jr. Serous retinal detachment in thrombotic thrombocytopenic purpura. Arch Ophthalmol 1985 Aug;103(8):1172-4.
53. Shin SY, Lee JM. A case of multiple cranial nerve palsies as the initial ophthalmic presentation of antiphospholipid syndrome. Korean J Ophthalmol 2006 Mar;20(1):76-8.
54. Miserocchi E, Baltatzis S, Foster CS. Ocular features associated with anticardiolipin antibodies: a descriptive study. Am J Ophthalmol 2001 Apr;131(4):451-6.
55. Lima Cabrita FV, Foster CS. Anticardiolipin antibodies and ocular disease. Ocul Immunol Inflamm 2005 Jul-Aug; 13(4):265-70.
56. Proia A, Paesano R, Torcia F, et al. Thrombotic thrombocytopenic purpura and pregnancy: a case report and a review of the literature. Ann Hematol 2002 Apr;81(4):210-4.
57. Elsherbiny SM, Benson MT. Retinal detachment and the second stage of labour: a survey of regional practice and literature review. J Obstet Gynaecol 2003 Mar;23(2):114-7.
58. Neri A, Grausbord R, Kremer I, et al. The management of labor in high myopic patients. Eur J Obstet Gynecol Reprod Biol 1985;19:277-9.
59. Landau D, Steelenfreund MH, Tadmor O, et al. The effect of normal childbirth on eye abnormalities predisposing to rhegmatogenous retinal detachment. Graefes Arch Clin Exp Ophthalmol 1995;233:598-600.
60. Prost M. Severe myopia and delivery. Klinica Oczna 1996;98:129-30.
61. Kent-Smith BT, Wilson GA. Management of rhegmatogenous retinal detachment in a full-term pregnancy. Clin Experiment Ophthalmol 2005 Feb;33(1):101.

