 |
A corneal assessment is a routine and integral part of any ocular examination. Its elaborate and unique organization gives the cornea the necessary transparency to refract and transmit light rays to the retina for vision. This also makes examination difficult—as the cornea is an inherently clear structure, its very nature poses a challenge in determining if corneal pathology is present. There are some tools that can facilitate assessment, one of those being the use of vital dyes.
One Dye, Many Uses
Of the available ophthalmic dyes, fluorescein is most commonly used for corneal evaluation. The agent, fluorescein sodium, is an orange-red hydrocarbon with low molecular weight.1 It is available in topical and intravenous forms for ocular use. For the evaluation of corneal pathology, it comes impregnated in a paper strip and dissolves when moistened with normal saline solution to be introduced onto the surface of the eye. This is done by placing the moistened strip in the inferior conjunctiva or fornix and having the patient blink multiple times to allow the agent to spread across the ocular surface.
One of its unique properties is that it reacts to wavelengths in the visible spectrum, ranging from 465nm to 490nm. It is most evident under cobalt blue light. Under normal lighting conditions, it appears orange on the ocular surface. However, on observation of fluorescein sodium under the cobalt blue filter, it fluoresces to wavelengths of 520nm to 530nm, appearing bright green to the observer. The charged ends of this compound are attracted to the hydrophilic ends of the cell membrane in the cornea, thus illuminating areas where cells are broken down or missing.2 These properties allow for its many ophthalmic uses:
• Evaporative dry eye assessment. The diagnosis of dry eye syndrome, particularly the evaporative form, is made possible through the use of a fluorescein sodium strip and slit lamp. A commonly used diagnostic test called tear breakup time is most optimally performed by placing a fluorescein strip onto the eye, waiting two minutes and then viewing the cornea with the cobalt blue filter under high illumination at 10x magnification. The patient is asked to blink once and then keep their eyes open as long as they can. Simultaneously, the examiner observes the cornea for the first appearance of a dry spot, which presents as a dark area among the otherwise uniformly green appearance of the cornea, and counts the number of seconds that it takes to manifest. Studies have indicated that a measurement of less than 10 seconds is abnormal.3,4
 |
|
Punctate epithelial erosions in a dry eye patient as revealed by fluorescein dye. (Image courtesy of W.R. Buie.) Click image to enlarge. |
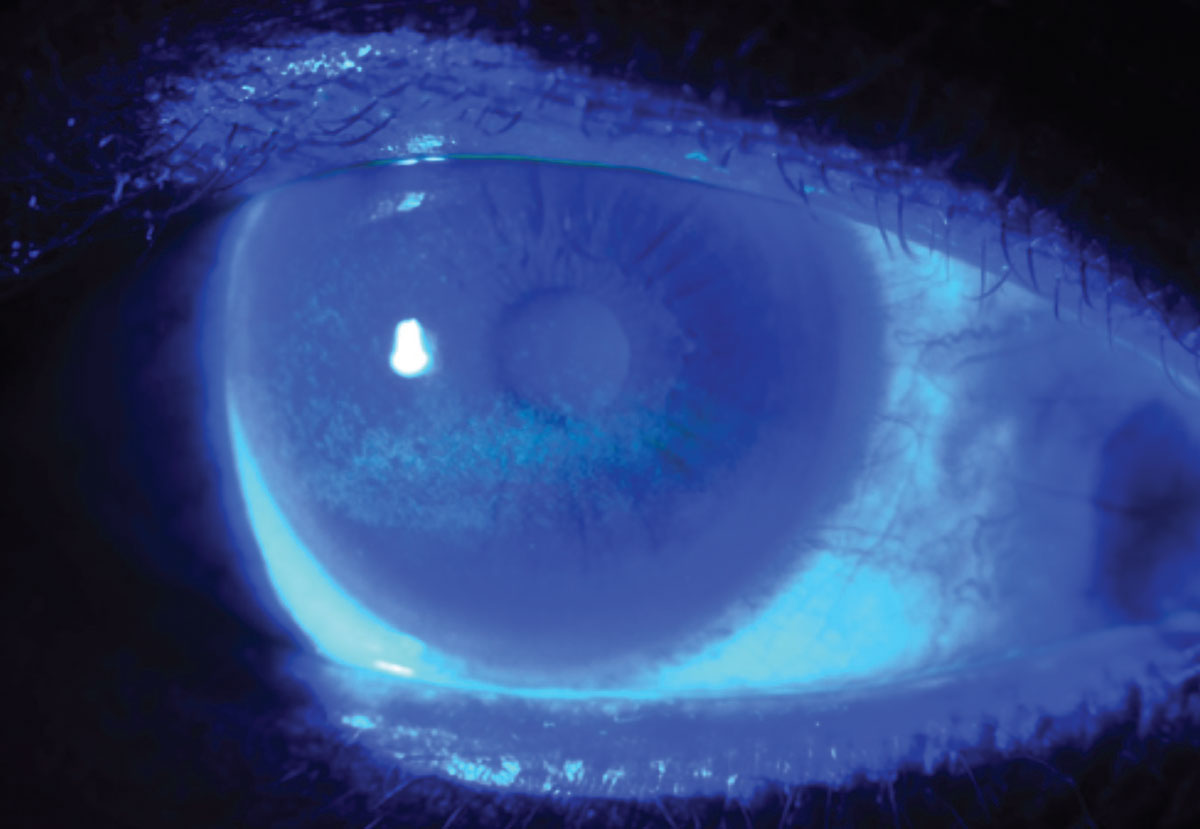
• Aqueous-deficient dry eye assessment. In more aqueous-deficient forms of dry eye syndrome, fluorescein can also be used to illuminate the presence, severity and course of dryness. This is done in a different manner than evaporative dry eye, in that the dye is inserted and observation of the cornea is best performed between four and eight minutes following instillation.
In these cases, the staining pattern of fluorescein, which appears as bright green punctate lesions on the cornea, is evaluated and given a score. These dot-like, stained green lesions are known as punctate epithelial erosions (PEEs). The absence of PEEs is given a score of zero. If one to five PEEs are observed, it is scored as grade one, six through 30 is scored as two and greater than 30 is given a score of three. Location of the staining and confluent areas are also noted. This observation method using fluorescein and a grading scale aids in determining the severity of dry eye disease, as well as in monitoring the efficacy of treatment.4

|
|
Superior central staining with fluorescein sodium dye, indicating a break in the corneal epithelium, viewed with a cobalt blue filter. Click image to enlarge. |
• Corneal abrasion. This is a routinely encountered condition, and its diagnosis is facilitated by the use of fluorescein dye. Since the dye stains between cells and any exposed areas of basement membrane, it is an easy way to identify epithelial defects of any size. The staining pattern may also provide information on the cause of the abrasion. For example, linear staining may indicate tracking from a foreign body.5
• Corneal ulcer. These are a common cause of permanent vision loss worldwide, requiring accurate and prompt diagnosis to preserve vision. The pattern of fluorescein staining is diagnostic, as it stains the overlying epithelial defect. The size of this defect in comparison to the size of the underlying infiltrate gives the practitioner information regarding the infectious nature of the condition, indicated by an equal one-to-one staining ratio.5,6
• Herpetic keratitis. Another type of infectious keratitis is that which occurs from herpes viruses. Though the precise diagnosis can be made using other ophthalmic dyes, such as rose bengal and lissamine green, the use of fluorescein can give a gross diagnosis of herpes keratitis. This is made through the pattern of staining, which has a characteristic branching, dendritic appearance.5,7
• Open globe. In the context of ocular injury, a necessary part of ocular examination is determining whether there is a defect in the globe. This is assessed through the Seidel test, which necessitates the use of fluorescein dye. The dye is instilled, and the cornea is observed under the usual cobalt blue light.
Under normal circumstances, the cornea displays a uniform, green appearance. In the case of a positive Seidel sign, aqueous percolates through the eye onto the corneal surface, streaming down like a waterfall due to gravity, thus diluting the fluorescein dye. This causes a dark linear stream in contrast to the bright green intact cornea. A positive Seidel test result means there is a full-thickness defect in the globe, which may otherwise be undetectable without the use of fluorescein.8 A similar principle is also used to evaluate bleb integrity in surgical glaucoma cases.9
• Contact lens evaluation. Fluorescein is an essential part of the fitting and evaluation of rigid or scleral contact lenses. Using this dye in conjunction with the lens gives the practitioner information regarding the fit, tear exchange dynamics and potential complications.10
Takeaways
The uses of topical fluorescein dye are numerous. It can even be combined with anesthetic for Goldmann applanation tonometry in measuring intraocular pressure. Fluorescein strips are a safe, readily available and cost-effective tool; as such, it is important to understand the dye’s unique properties and capabilities to optimize diagnosis and treatment.2
Dr. Labib graduated from Pennsylvania College of Optometry, where she now works as an associate professor. She completed her residency in primary care/ocular disease and is a fellow of the American Academy of Optometry and a diplomate in the Comprehensive Eye Care section. She has no financial interests to disclose.
1. Das D, Deka P, Bhattacharjee H, et al. Fluorescein dye as a novel cost-effective approach for staining raw specimens in ophthalmic pathology. Indian J Ophthalmol. 2020;68(10):2175-8. 2. Pothen AG, Parmar M. Fluorescein. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. 3. Paugh JR, Tse J, Nguyen T, et al. Efficacy of the fluorescein tear breakup time test in dry eye. Cornea. 2020;39(1):92-8. 4. Whitcher JP, Shiboski CH, Shiboski SC, et al. A simplified quantitative method for assessing keratoconjunctivitis sicca from the Sjögren’s Syndrome International Registry. Am J Ophthalmol. 2010;149(3):405-15. 5. Wipperman JL, Dorsch JN. Evaluation and management of corneal abrasions. Am Fam Physician. 2013;87(2):114-20. 6. Loh K, Agarwal P. Contact lens related corneal ulcer. Malays Fam Physician. 2010;5(1):6-8. 7. Pflipsen M, Massaquoi M, Wolf S. Evaluation of the painful eye. Am Fam Physician. 2016;93(12):991-8. 8. Campbell TD, Gnugnoli DM. Seidel test. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. 9. Akiyama G, Saraswathy S, Bogarin T, et al. Functional, structural, and molecular identification of lymphatic outflow from subconjunctival blebs. Exp Eye Res. 2020;196:108049. 10. Muntz A, Subbaraman LN, Sorbara L, et al. Tear exchange and contact lenses: a review. J Optom. 2015;8(1):2-11. |

