With spring almost here, we must once again start to think about warm weather, blooming flowers, green grass and the inevitable surge in cases of ocular allergy. But let’s not neglect those patients who suffer year-round from perennial allergies secondary to pet dander, dust and mold. We must also be proactive with our case histories, asking just the right questions to prevent needless patient suffering or self-treatment from the pharmacy or their own medicine cabinets. In this article we will review the etiology, diagnosis and the most current treatment of this insidious and prevalent condition.
Little Itch, Big Problem
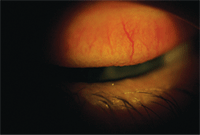
Allergic papillary conjunctivitis.
Approximately 20% of the population in the United States suffers from allergies, and ocular involvement is estimated to be present in 40% to 60%, accounting for tens of millions of people.1
Although ocular allergy is not typically a sight-threatening condition, it can disrupt an individual’s quality of life. Allergy symptoms can affect a patient’s ability or willingness to spend time outdoors, read or drive, as well as their capacity to read and concentrate on a task, thus impacting home, school, recreational and work lives. Indeed, allergic rhinitis imposes a substantial economic burden on society that manifests as absence from work and reduced working capacity and is estimated to be in the billions of dollars.2
A survey by the Asthma and Allergy Foundation of America (AAFA) showed that approximately 40% of female allergy sufferers claim that their red and puffy eyes make them look tired and unattractive. This can be attributed to their inability to wear contact lenses and/or makeup.3 Prescribing physicians do not believe that patients are overstating their symptoms, but family members and co-workers often dismiss allergies as insignificant, which can take an emotional toll.4
Inside the Allergic Response
Allergy is a hypersensitivity reaction of the immune system to an otherwise innocuous substance or environmental allergen that the body identifies as hazardous.
Allergy commonly affects various target organs including the eyes, nose, sinuses, ears, lungs and skin.
Ocular allergy can be subdivided into two main categories; the more frequent, but mild, seasonal allergic conjunctivitis (SAC) and perennial allergic conjunctivitis (PAC), with its symptoms of itching and redness, and the less frequent, but more serious, sight-threatening vernal keratoconjunctivitis (VKC) and atopic keratoconjunctivitis (AKC).5
• SAC/PAC. In SAC and PAC, the interaction between the allergen and the immunoglobulin E (IgE) antibodies that reside on sensitized mast cells allows for an influx of calcium ions, causing mast cell degranulation, which releases preformed mediators, most notably histamine. Histamine increases vascular permeability, which causes edema in the surrounding tissue and yields the puffy, itchy, red eyes that signify ocular allergies.6 This is the early phase of the Type 1 hypersensitivity response.
This early phase response peaks at approximately 20 minutes post-allergen exposure. The late-phase response involves mast cells, T cells and eosinophils, and peaks at approximately six hours post-allergen exposure. SAC is thought to be primarily mast cell driven; however, in addition to the mast-cell response, output of cytokines by conjunctival epithelium T cells leads to multiple cytokine appearance in tears in SAC.7
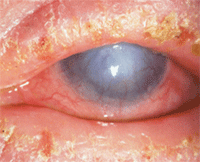
Allergic blepharitis and dermatitis associated with AKC.
• VKC/AKC. VKC and AKC involve chronic mast cell activation and an eosinophil/T-lymphocyte-mediated late phase, causing a release of cytokines and interferon.
What distinguishes VKC from SAC and PAC is this tremendous infiltration of inflammatory cells and its effect on the cornea. AKC is the ocular component of an altered systemic condition that includes atopic dermatitis and asthma.8
There are four main histamine receptors in the body: H1 receptors, which have been the main site of antihistamine agent activity, mediate contraction of smooth muscle and capillary dilation; H2 receptors mediate acceleration of heart rate and promotion of gastric acid secretion; H3 receptors are believed to play a role in regulation of the release of histamine and other neurotransmitters from neurons; H4 receptors are located in the basophils, bone marrow, thymus, small intestine, spleen and colon, and play a role in chemotaxis, the movement of body cells in reaction to a chemical in their environment.9
For the purposes of this article, we will focus on the current topical treatment for seasonal and perennial allergic conjunctivitis.
Food and Allergy
Interestingly, there may be an association between food consumption and allergy development. In a review of more than 62 studies, adherence to a Mediterranean diet, which consists of fish and vegetables, was found to be protective for wheezing and atopy.10 A significant number of fruit and vegetable studies have reported beneficial associations with asthma and allergy developments.11,12
Alternatively, perhaps it is a reduction in the consumption of these food products that is helping to spur the increased allergy rates being noted.13 Researchers found a weak association between the consumption of apples and fish during pregnancy, and speculate that these foods offer a protective effect against the development of asthma and allergic disease.14 Another study found similar results with diets of fruit, vegetables and fish being associated with lower rates of wheeze and allergy.10
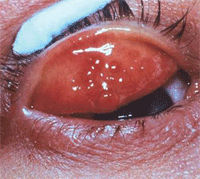
Cobblestone papillae associated with VKC.
Non-pharmacological Treatment
The key element to non-medical treatment is avoidance of the known allergen and/or the use of palliative remedies to minimize symptoms. But this may be difficult, if not downright impractical. In order to reduce contact with known allergens, patients should stay indoors on days with high pollen counts and use air conditioning rather than keep their windows open. They must avoid freshly cut grass, remove pets from their bedrooms and keep their living areas as dust free as possible. They can be advised to wash their hands and face as well as brush their hair when they come indoors to diminish the spread of allergens.
Palliative measures include over-the-counter (OTC) non-preserved lubricating drops, cool compresses and cold packs. These therapies aid in vasoconstriction and diminish lid swelling. Discourage patients from eye rubbing because this action can mechanically degranulate mast cells.
If this form of treatment proves ineffective, it should be complemented with anti-allergy pharmaceutical agents.
Pharmacological Treatment
• OTC decongestants and decongestant/antihistamine combination agents. Decongestants, such as Visine LR (oxymetazoline hydrochloride, Johnson & Johnson),
Visine (tetrahydrozoline hydrochloride, Johnson & Johnson), Clear Eyes (naphazoline hydrochloride, Prestige Brands) and Refresh Redness Relief (phenylephrine hydrochloride, Allergan) are primarily sought out by patients as a quick, non-prescription, cosmetic fix. They have no effect on histamine receptors but merely counteract the histamine-mediated redness and erythema through vasoconstriction.
While they act quickly, their duration is short-lived, so patients tend to dose frequently, often causing rebound hyperemia, acute and chronic conjunctivitis, medicamentosa and the release of inflammatory mediators from mast cells.11 These drops can induce pupillary dilation, so should be avoided in patients with at-risk anterior chamber angles.12
Decongestants are paired with antihistamines in the following products; Opcon-A (naphazoline HCl 0.03%/pheniramine maleate 0.32%, Bausch + Lomb), Naphcon-A (naphazoline HCl 0.025%/pheniramine maleate 0.3%, Alcon Pharmaceuticals) and Visine-A (naphazoline HCl 0.025%/pheniramine maleate 0.3%, Johnson & Johnson).
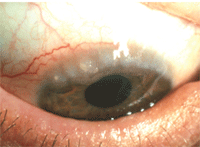
Trantas dots associated with VKC.
• Antihistamines. Historically, antihistamines competitively bind to H1 receptors, preventing the histamine released from mast cells from binding, thus reducing itching, while the blockade of the H1 receptors on endothelial smooth muscle tissues on blood vessels relieves redness and swelling. For the purposes of this article, we will avoid discussion of systemic antihistamines because they do not target ocular tissues and can cause dryness of the tear film, which can lead to retention of inflammatory mediators.15 Instead, we’ll focus our attention on topical treatment.
Topical antihistamines, while safe and effective, encounter compliance difficulty secondary to their short duration of action and q.i.d. dosing.16 Livostin (levocabastine hydrochloride 0.05% ophthalmic suspension, CIBA Vision) was discontinued in 2006, but Emadine (emedastine difumarate, Alcon) ophthalmic solution 0.05% is still available.
The recent FDA approval of Lastacaft (alcaftadine 0.25%, Allergan) represents a new molecular entity in the field of antihistamine allergy agents. It is a mixed mechanism antihistamine that acts as an H1, H2 and H4 antagonist. The serendipitous discovery of histamine H4 receptors occurred when scientists were investigating histamine H3 receptors in brain cells.17 These H4 receptor antagonists may have therapeutic use for treating pruritic diseases where histamine H1 receptor antagonists are not effective.18
Alcaftadine offers anti-inflammatory properties that allow stabilization of conjunctival epithelial tight junctions, prevents vascular leakage, and inhibits early- and late-phase allergic inflammation. In a multicentered, placebo controlled Phase III study of 58 subjects, alcaftadine was found to be more effective in the prevention of ocular itching, with a duration of action within three minutes and a duration of action of at least 16 hours.19 It is approved for ages two and up and dosed just once daily.20
• Mast cell stabilizers. Mast cell stabilizers suppress mast cell degranulation. Their popularity of use is limited by two major factors: they require a minimal loading time of two weeks because they have no effect on allergic reactions caused by previously released histamine and most require q.i.d. dosing, so they are not a good option for early-stage hypersensitivity reactions such as seen in SAC and PAC.
In a 2005 Harris Interactive Poll of 1,214 adults, 85% of allergy patients believed it important that prescription medications work quickly, and 44% were dissatisfied with the time it took their current medication to relieve symptoms.3 Current mast cell stabilizers include Alomide (lodoxamide tromethamine, Alcon), Alamast 0.1% (pemirolast potassium, Vistakon Pharmaceuticals), Crolom (cromolyn sodium 4%, Bausch + Lomb) and Alocril (nedocromil sodium 2%, Allergan).21
• Combination agents. Topical antihistamine/mast cell stabilizers have become the gold standard of treatment because of their dual mode of action. They competitively block histamine binding to H1 and/or H2 receptors, providing rapid relief of symptoms, as well as inhibiting mast cell degranulation.
The first dual-action drug, Patanol (olopatadine 0.1%, Alcon) was introduced in 1997. Pataday, introduced in 2004 with twice the concentration of olopatadine, can be dosed just once daily. The other combination products, Optivar (azelastine, Meda Pharmaceuticals), Elestat (epinastine 0.05%, Inspire Pharmaceuticals) and ketotifen are approved only for itching associated with allergic conjunctivitis.
With the transition of Zaditor (ketotifen fumarate 0.025%, Novartis) to OTC status in late 2006, patients are now able to purchase an effective combination antihistamine-mast cell stabilizer without a prescription and often at a lower cost. Other OTC ketotifen eye drops include: Alaway (Bausch + Lomb), Claritin Eye (Schering-Plough), Refresh Eye Itch Relief (Allergan) and Visine All Day Eye Itch Relief (Johnson & Johnson). Zyrtec Itchy Eye Drops (Johnson & Johnson) is currently off the market after a voluntary recall in March 2010.
Bepreve (bepotastine besilate 1.5%, Ista Pharmaceuticals) was approved by the FDA in September 2009 for the treatment of ocular itching associated with allergic conjunctivitis for ages two years and up. It is an antihistamine-mast cell stabilizer combination drug that is highly specific to H1 receptor antagonists, as well as inhibiting other cytokines in the inflammatory cascade. Bepotastine has demonstrated rapid and sustained reductions in ocular itching but not conjunctival hyperemia.22 Bepotastine was found to be more potent than olopatadine, ketotifen and levocabastine in reducing vascular hyperpermeability in various animal models of allergic conjunctivitis. The researchers suggest that bepotastine inhibits the allergic response through several mechanisms—histamine H1 receptor antagonism, mast cell stabilization and inhibition of eosinophil migration to ocular inflammatory sites.23
• Non-steroidal anti-inflammatories. NSAIDs block the cyclo-oxygenase pathway and the formation of prostaglandins. These drugs have no effect on histamine, thus limiting their value in the treatment of SAC or PAC. Acular (ketorolac 0.5%, Allergan), has been proven safe when used on a long-term basis (>12 months) for the treatment of seasonal and perennial allergic conjunctivitis.24 Voltaren (diclofenac sodium 0.1%, Novartis) has demonstrated similar effect on the signs and symptoms.25 However, as with other topical therapies, NSAIDs are known to cause discomfort on instillation, which may affect patient compliance.15
• Corticosteroids. Corticosteroids are not typically used for long-term treatment of SAC and PAC because they do not block histamine release and have potential complications, including cataract formation, increased intraocular pressure and decreased wound-healing time. Alrex (loteprednol etabonate ophthalmic suspension 0.2%, Bausch + Lomb) was the first site specific “soft” steroid approved for the short-term treatment of allergic conjunctivitis, though it has been shown to be safe when used for longer than 12 months.26 Successful prophylactic treatment with loteprednol 0.5% has also been demonstrated due to its superior safety profile over ketone corticosteroids and may be a good option when chronic treatment is necessary.27,28
The ketone steroids, such as prednisolone acetate, are typically reserved for the more serious inflammatory based VKC and AKC, as they suppress the mediators released in the late phase of ocular allergic response.
• Immunomodulators. Cyclosporine eye drops, at 1% and 2% concentrations, have been shown to be safe and effective for the long-term treatment of VKC in 156 children, and as an alternative to topical corticosteroids and their potential side effects with long-term use.29 In one study, while it proved safe and effective for the treatment of AKC, there was marked blurring of vision and intense stinging after drop instillation, which can limit patient compliance.30
Currently, cyclosporine 0.05% is only FDA approved to increase tear production in patients whose tear production is presumed to be suppressed due to ocular inflammation associated with keratoconjunctivitis sicca.31
Relief for All Patients
Now more than ever, we have the opportunity to help a growing number of our patients overcome the physical, emotional and financial problems of ocular allergies.
With the recent additions to our therapeutic armamentarium, we can offer new ways to stop the symptoms almost before they begin, allowing our patients to live healthy and productive lives.
But first we must educate our patients on what to do if confronted with ocular allergy symptoms and when to follow up with their eye care practitioner if palliative therapies do not provide quick or effective relief.
Dr. Chaglasian is an Associate Professor of Optometry at the Illinois College of Optometry in Chicago and an associate at Mack Eye Center, a cornea referral practice in Hoffman Estates, Ill.
1. Ono SJ, Abelson MB. Allergic conjunctivitis: update on pathophysiology and prospects for future treatment. J Allergy Clin Immunol. 2005 Jan;115(1):118-22.
2. Alexander M, Berger W, Buchholz P, et al. The reliability, validity, and preliminary responsiveness of the eye allergy patient impact questionnaire (EAPIQ). Health Qual Life Outcomes. 2005 Oct 31;3:67.
3. Hellgren J, Cervin A, Nordling S, Bergman A, Cardell LO. Allergic rhinitis and the common cold – high cost to society. Allergy. 2010 Jun 1;65(6):776-83.
4. AAFA. Eye Allergy Survey Results. Available at: www.aafa.org/display.cfm?id=7&sub=100&cont=688. (Accessed January 2011).
5. New Survey Suggests Cultural Ambivalence To Allergies Leaves Many Suffering Needlessly. Available at: www.medicalnewstoday.com/articles/101313.php. (Accessed January 2011).
6. Von Mutius E. Allergies, infections and the hygiene hypothesis – the epidemiological evidence. Immunobiology. 2007;212(6):433-9.
7. Ariano R, Canonica GW, Passalacqua G. Possible role of climate changes in variations in pollen seasons and allergic sensitizations during 27 years. Ann Allergy Asthma Immunol. 2010 Mar;104(3):215-22.
8. Foster CS, Calonge M. Atopic keratoconjunctivitis. Ophthalomology 1990;97:992-100.)
9. D’Amato G, Cecchi L, D’Amato M, Liccardi G. Urban air pollution and climate change as environmental risk factors of respiratory allergy: an update. J Investig Allerg Clin Immunol. 2010;20(2):95-102.
10. Nurmatov U, Devereux G, Sheikh A. Nutrients and foods for the primary prevention of asthma and allergy: Systematic review and meta-analysis. J Allergy Clin Immunol. 2010 Dec 23. [Epub ahead of print]
11. Willers SM, Devereux G, Craig LC, et al. Maternal food consumption during pregnancy and asthma, respiratory and atopic symptoms in 5-year-old children. Thorax. 2007 Sep;62(9):773-9.
12. Chatzi L, Torrent M, Romieu I, et al. Diet, wheeze, and atopy in school children in Menorca, Spain. Pediatr Allergy Immunol. 2007 Sep;18(6):480-5.
13. Reid CE, Gamble JL. Aeroallergens, allergic disease, and climate change: impacts and adaptation. Ecohealth. 2009 Sep;6(3):458-70.
14. Leonardi A, Lanier B. Urban eye allergy syndrome: a new clinical entity? Curr Med Res Opin. 2008 Aug;24(8):2295-302.
15. http://Torkildsen G, Shedden A The safety and efficacy of alcaftadine 0.25% ophthalmic solution for the prevention of itching associated with allergic conjunctivitis. Curr Med Res Opin. 2011 Mar;27(3):623-31. Epub 2011 Jan 21.
16. Leonardi A. Emerging drugs for ocular allergy. Expert Opin Emerg Drugs. 2005 Aug;10(3):505-20.
17. Stock EL, Rorh SI, Kim ED, Walsh MK, Thamman R. The effect of platelet-activating factor (PAF), histamine, and ethanol on vascular permeability of the guinea pig conjunctiva. Invest Ophthalmol Vis Sci. 1990 May;31(5):987-92.
18. Anderson DF, MacLeod JD, Baddeley SM, et al. Seasonal allergic conjunctivitis is accompanied by increased mast cell numbers in the absence of leucocyte infiltration. Clin Exp Allergy. 1997 Sep;27(9):1060-6.
19. Zampeli E, Tiligada E. The role of histamine H4 receptor in immune and inflammatory disorders. Br J Pharmacol. 2009 May;157(1):24-33.
20. Soparkar CNS, Wilhelmus KR, Koch DD, Wallace GW, Jones DB. Acute and chronic conjunctivitis due to over-the-counter decongestants. Arch Ophthalmol. 1997
Jan;115(1):34-8.
21. Abelson MB, Yamamoto GL, Allansmith MR. Effects of ocular decongestants. Arch Ophthalmol. 1980 May;98(5);856-8.
22. Ousler GW, Wilcox KA, Gupta G, Abelson MB. An evaluation of the ocular drying effects of 2 systemic antihistamines; loratadine and cetrizine hydrochloride. Ann Allergy Asthma Immunol. 2004 Nov;93(5):460-4.
23. van Cauwenberge P, Bachert C, Passalacqua G, et al. Consensus statement on the treatment of allergic rhinitis. Allergy. 2000 Feb;55(2):116-34.
24. Bhatt HG, Agrawal YK, Raval HG, Manna K, Desai PR. Histamine H4 receptor: A novel therapeutic target for immune and allergic responses. Mini Rev Med Chem. 2010 Dec 1;10(14):1293-308.
25. Dunford PJ, Williams KN, Desai PJ, et al. Histamine H4 receptor antagonists are superior to traditional antihistamines in the attenuation of experimental pruritus. J Allergy Clin Immunol. 2007 Jan;119(1):176-83.
26. Ilyas H, Slonim CB, Braswell GR, et al. Long-term safety of loteprednol etabonate 0.2% in the treatment of seasonal and perennial allergic conjunctivitis. Eye Contact Lens. 2004 Jan;30(1):10-3.
27. Dell SJ, Shulman DG, Lowry GM, Howes J. A controlled evaluation of the efficacy and safety of loteprednol etabonate in the prophylactic treatment of seasonal allergic conjunctivitis. Loteprednol Allergic Conjunctivitis Study Group. Am J Ophthalmol. 1997 Jun;123(6):791-7)
28. Pavesio CE, Decory HH. Treatment of ocular inflammatory conditions with loteprednol etabonate. Br J Ophthalmol 2008;92(4):455-9)
29. Ozcan AA, Ersoz TR, Dulger E. Management of severe allergic conjunctivitis with topical cyclosporin a 0.05% eyedrops. Cornea. 2007 Oct;26(9):1035-8)
30. Hingorani M, Moodaley L, Calder VL, Buckley RJ, Lightman S. A randomized, placebo-controlled trial of topical cyclosporin A in steroid-dependent atopic keratoconjunctivitis. Ophthalmology. 1998 Sep;105(9):1715-20).
31. Restasis Package Insert, Allergan, Inc
32. Alocril Package Insert, Allergan, Inc. Irvine, CA.
33. Abelson AB,Torkildsen GL,Williams JI, et al. Time to onset and duration of action of the antihistamine bepotastine besilate ophthalmic solutions 1.0% and 1.5% in allergic conjunctivitis: a phase III, single-center, prospective, randomized, double-masked, placebo-controlled, conjunctival allergen challenge assessment in adults and children. Clin Ther. 2009 Sep;31(9):1908-21.

