The DRCR Retina NetworkFormed in 2002, this collaborative group is dedicated to clinical research applicable to improving patient care. The network originally focused on diabetic retinopathy research, but since 2018 has expanded their research to all retinal disorders. DRCR protocols, past and present, can be accessed at public.jaeb.org/drcrnet. |
As always, an extensive number of promising clinical trials are underway in the retina world. Much of the current focus is on therapeutic development and drug delivery systems, with the ultimate goal of reducing the treatment burden for those receiving anti-VEGF. Many of the therapeutics in the pipeline—mainly injectables—will have minimal direct influence on an optometrist’s role, as those patients still need a referral, but they could substantially change the patient’s experience at the retina specialty clinic that we prepare them for at the outset of care. So, it’s incumbent on us to be as up to date as possible even on interventions we do not personally administer.
More directly affecting our own protocols, there are also a number of current and completed clinical trials of particular interest to ODs that would allow us to keep patients in the chair longer, have an active role in treatment or change our referral patterns.
Most of the focus is on diabetic eye disease and macular degeneration, but there are also exciting prospects for inherited retinal dystrophies. Here are several trials, active or recently completed, with the potential to influence our clinical practice.
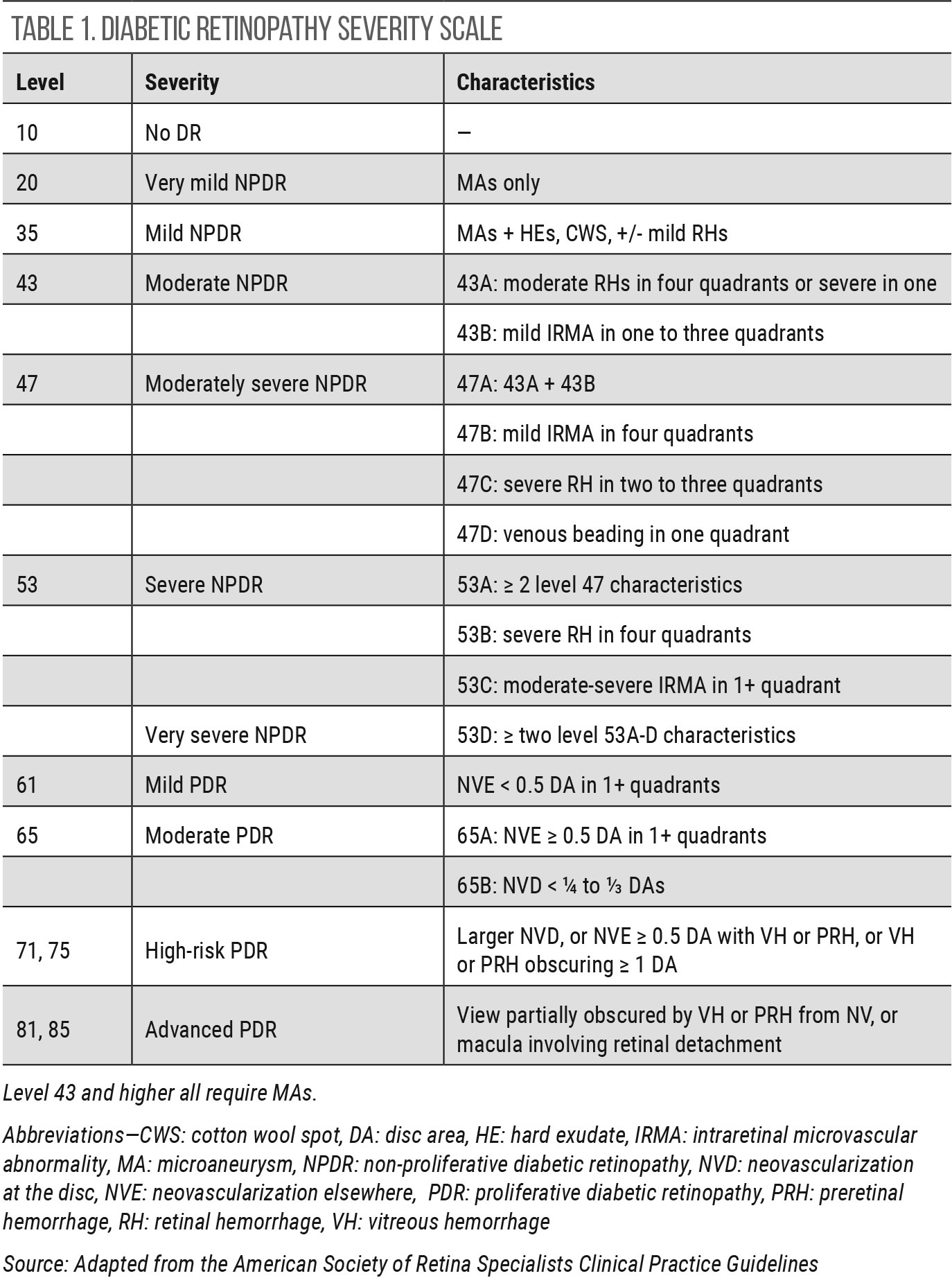 |
| Click table to enlarge. |
Nonproliferative Diabetic Retinopathy
There may well be no topic in retina more deserving of attention from researchers than diabetic eye disease, especially in its earliest stages, given the pervasiveness of diabetes and its unfortunately inevitable increase in prevalence in the coming years.
• DRCR Protocol W
Status: in progress
Anticipated completion: May 2022
• PANORAMA
Status: complete
The goal with diabetic retinopathy (DR) certainly is to preserve vision. But when is intervention necessary? Historically, only eyes with proliferative diabetic retinopathy (PDR) or diabetic macular edema (DME) have been treated, and optometrists have referred those patients to retina specialists. Protocol W is looking at those patients with severe nonproliferative diabetic retinopathy (NPDR) to see if earlier intervention leads to a better outcome; specifically, whether it prevents progression to PDR and/or center-involved DME (CI-DME).1,2
The study boasts 328 participants with half randomized to sham injection and the other half to aflibercept injection.1,2 In the trial, aflibercept is dosed at months one, two and four, and then every four months thereafter. At the two-year mark, dosing will be decided as needed by the examining investigator.1,2
Protocol W is not the first prospective study to look into treating NPDR. The PANORAMA study demonstrated an impressive two-step reduction in Diabetic Retinopathy Disease Severity Scale (DRSS) for 58% of aflibercept-treated participants at six months.3,4 Unlike Protocol W, PANORAMA included eyes with both moderately severe NPDR (DRSS level 47) and severe NPDR (DRSS level 53). PANORAMA included two different treatment groups: Q8-week aflibercept dosing and Q16-week.3,4
With the two-year results of PANORAMA, announced in spring 2020, additional questions arose regarding treatment frequency. For study year two, the Q16-week treatment group maintained a two or more step improvement.5 However, the Q8-week group, when switched at week 52 to PRN dosing, showed a decline from 80% to 50% with two or more step improvement.5
The PANORAMA results unquestionably support earlier treatment in NPDR, but there are still lingering questions, such as how frequently to treat and when anti-VEGF injections can be stopped or tapered.
Clinical take home: Change in management of moderately severe and severe NPDR is on the horizon. Consider referral for patients that fall into either category.
• DRCR Protocol AF
Status: enrolling
Anticipated completion: January 2027
The DRCR recently announced the start of a Phase III clinical trial called Protocol AF that will study the effect of fenofibrate on NPDR.6,7 Fenofibrate is a peroxisome proliferator-activated receptor alpha agonist used to treat hypercholesterolemia by lowering triglycerides and low density lipoprotein and increasing high density lipoprotein.8
Fenofibrate’s positive effect on DR was first recognized by the FIELD study, which showed the number of patients on fenofibrate that went on to need laser treatment for DR or DME was significantly lower compared to controls.9 Additionally, the ACCORD study found a lower rate of NPDR progression in those taking fenofibrate plus simvastatin compared to the placebo plus simvastatin group.10
Neither the FIELD or ACCORD studies were primarily focused on the outcome of DR progression, which makes Protocol AF unique. Even though this study is still in the enrollment phase, it is worthy of being on optometrist’s radar as this is a potential means for our involvement—whether in comanagement or direct treatment roles—in an intervention for NPDR. Protocol AF seeks to enroll 910 participants with NPDR (without CI-DME) to follow over four years.6,7 Participants will be randomized to once daily fenofibrate 160mg or placebo.6,7
Clinical take home: Fenofibrate is an oral treatment under investigation for mild to moderately severe NPDR.
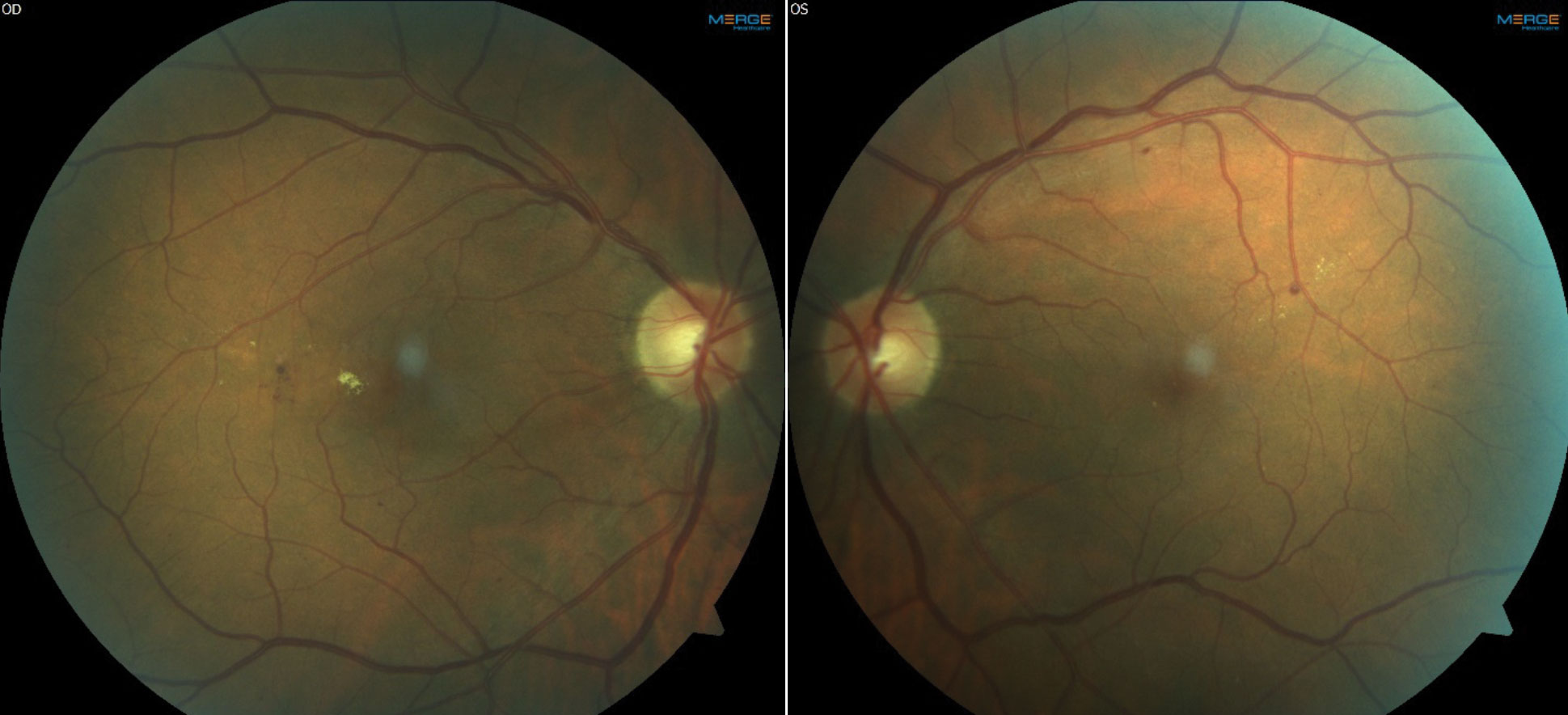 |
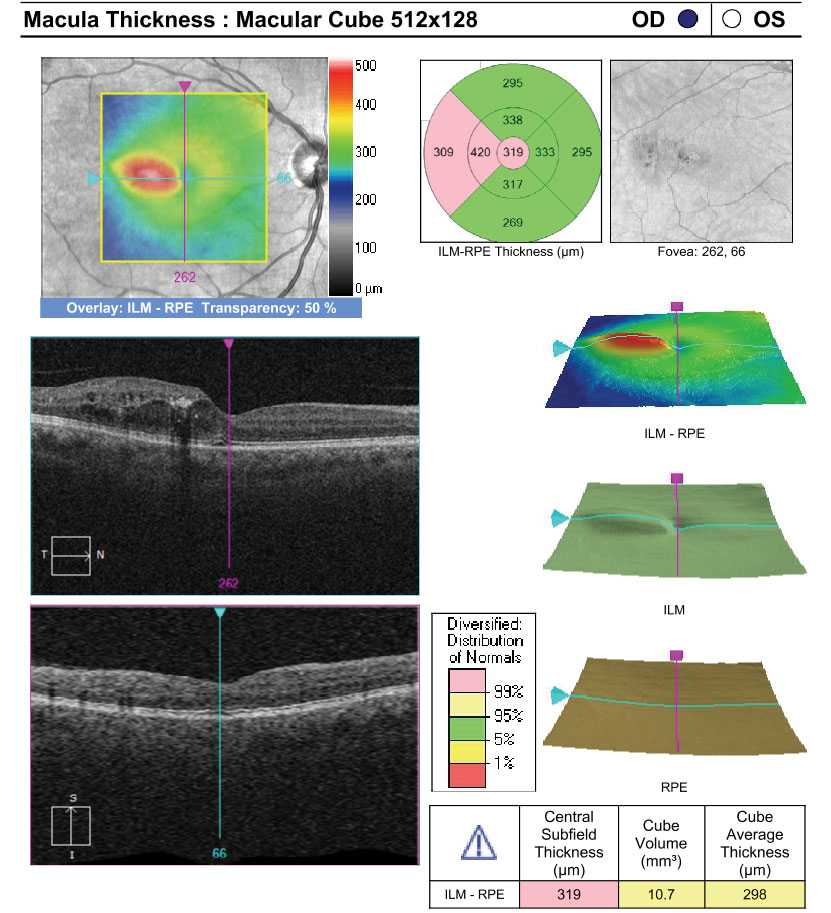 |
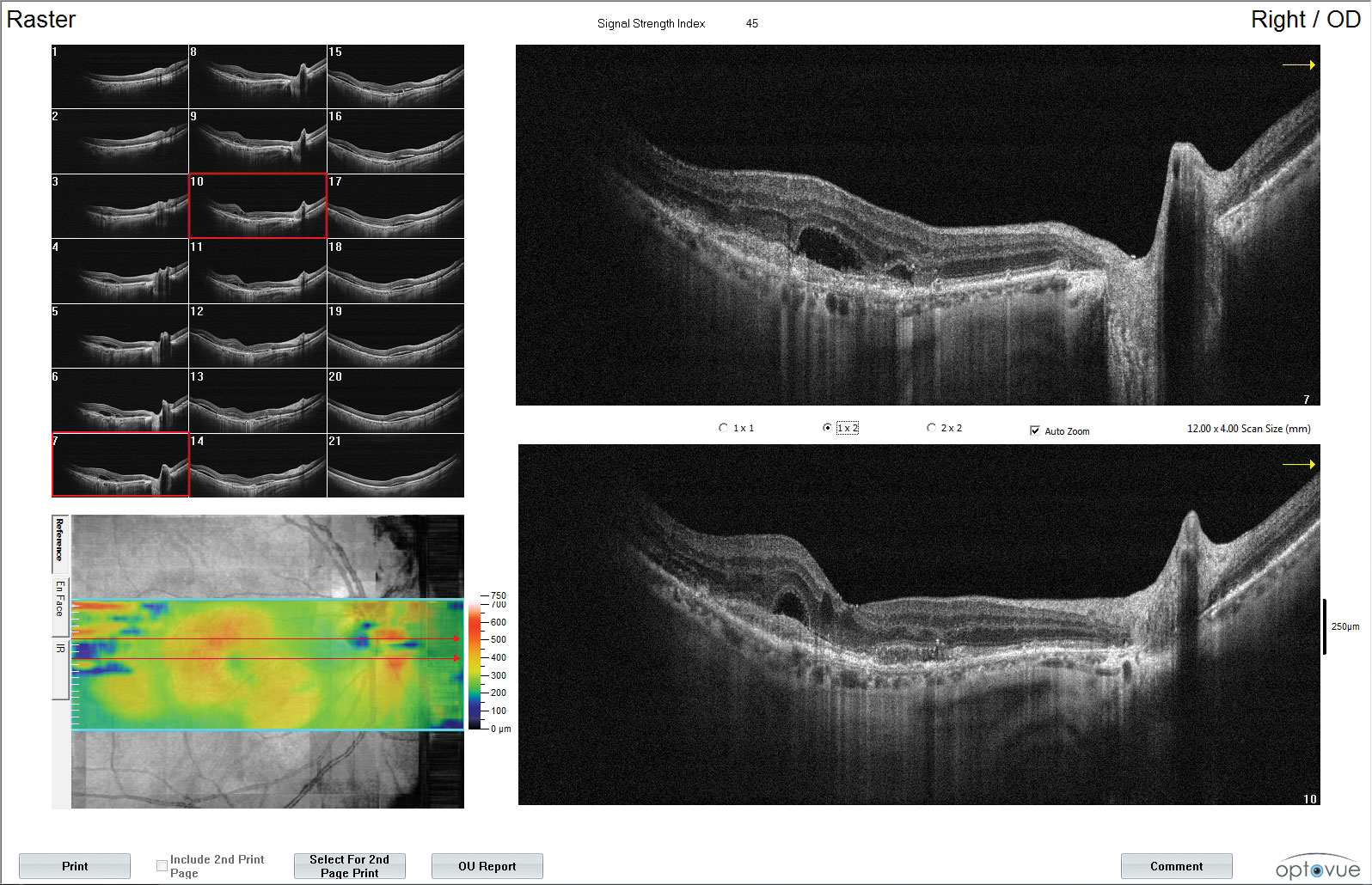
| Fundus images and OCT of a patient with moderate NPDR OU and CI-DME OD. The patient’s best corrected acuities were 20/25 OD and 20/20 OS. The comanaging retina specialist elected to monitor the CI-DME OD based on the findings of DRCR Protocol V. Click images to enlarge. |
Diabetic Macular Edema
Recalcitrance to treatment makes DME particularly nettlesome, but new treatment protocols offer hope for substantial improvement.
• DRCR Protocol V
Status: complete
In the current era, with OCT at our fingertips, DME is assessed as either center involved or non-CI, depending on the presence of thickening within the central subfield zone at the fovea. This is a change from the historical means of looking for characteristics of clinically significant macular edema (CSME). But what about patients who have mild CI-DME and a preserved visual acuity? Protocol V proved instrumental in providing guidance, as it assessed CI-DME eyes with 20/25 vision or better.11
A total of 702 participants were randomized to observation, treatment with aflibercept or treatment with laser photocoagulation.11,12 After two years, the study found no significant difference in visual acuity between each treatment groups and observation.11,12 The patients in the observation group that went on to decline in visual acuity were ultimately treated with aflibercept.12
Even though this trial has been completed and published, it is still not widely known among ODs, who have to pull the trigger on referral. It is further challenging for patients alike who have good visual acuities with minimal visual complaint and are not ready to be locked into a sometimes-endless cycle of injections.
Clinical take home: Patients with CI-DME and a visual acuity of 20/25 or better can be safely observed. This warrants a discussion with your local retina specialists to see if these patients necessitate a referral or can continue observation by you.
• YOSEMITE, RHINE
Status: in progress
Anticipated completion: summer/fall 2021
A new therapeutic that allows for fewer injections would be quite exciting news for our patients, particularly those in rural areas who may not be able to easily get to a retina specialist. Anti-VEGF therapy has done wonders for DME and wet AMD, but it comes at a price: frequent office visits impose a time burden on patients and their caregivers, and its cost weighs heavily on the healthcare system.
Faricimab (Roche) could be a game-changer. It’s not just another anti-VEGF treatment but rather a bispecific antibody that targets two pathways: VEGF-A and angiopoietin-2.13 The Phase III clinical trial data, released in February, was positive, showing half of faricimab-treated individuals were able to be dosed every four months.13 The YOSEMITE and RHINE trials are investigating faricimab in the treatment of CI-DME, with each trial including over 900 participants.14,15 There are sister trials underway for wet AMD (TENAYA and LUCERNE) that anticipate completion in 2022.
Clinical take home: Faricimab is an investigational drug targeting two pathways with the potential to be dosed less frequently than traditional anti-VEGF.
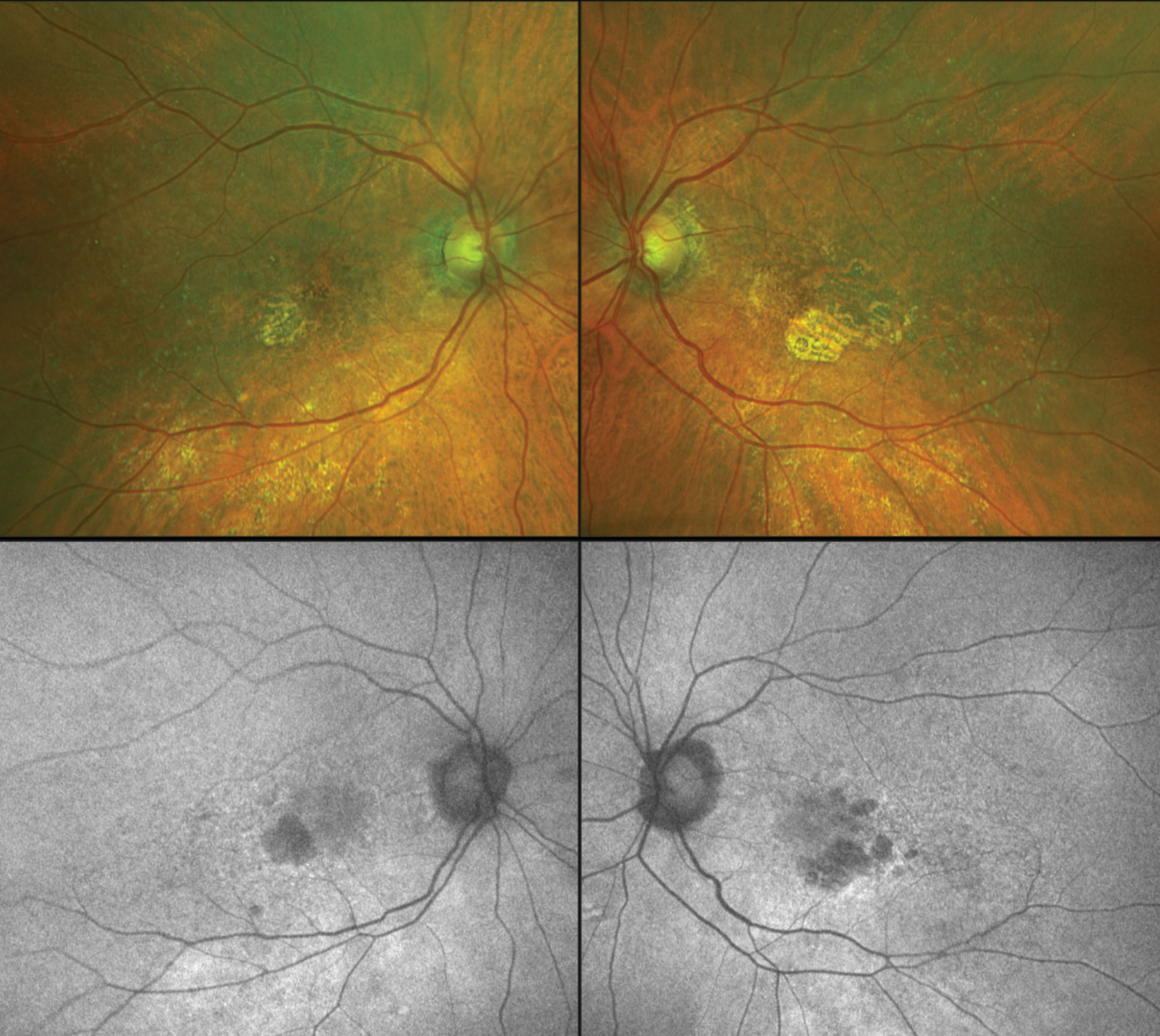 |
| Color fundus images (top) and fundus autofluorescence (bottom) of a patient with geographic atrophy (GA) from macular degeneration OU. The SAGA study is exploring modified vitamin A treatment for GA patients such as seen here. Click image to enlarge. |
Dry Macular Degeneration
One of the toughest nuts to crack in retina, dry AMD may be on the verge of breakthroughs that could finally offer more direct interventions.
• SAGA
Status: in progress
Anticipated completion: March 2022
Even though many therapeutics have been explored, none to date have been successful in slowing the progression of geographic atrophy (GA), much to the frustration of patients and optometrists alike.
Currently, there a few oral treatments in trial that, if successful, could bring optometrists into the treatment protocol. SAGA is a Phase II/III multicenter placebo-controlled clinical trial investigating the role of ALK-001 (Alkeus) in patients with GA.16
This drug is a modified form of vitamin A taken daily as an oral capsule. Over time, aggregates of vitamin A create dimers that accumulate in the RPE and underlying Bruch’s membrane.16 The dimers are thought to be toxic to the retina, which has been proposed as a mechanism for the AMD development.17 Synthetic ALK-001 contains vitamin A with deuterium, which slows formation of vitamin A dimers without compromising the normal function of the visual cycle.17
The primary outcome of the study is to assess the growth rate of GA lesions over a two-year period in those taking ALK-001 compared to placebo.16 If ALK-001 proves to be effective in slowing the progression of atrophy, it could provide a new treatment option to help reduce vision loss in those with geographic atrophy from AMD.
Additionally, Stargardt’s disease is caused by a mutation in the ABC4 gene, which affects the processing of vitamin A.18 This also leads to the accumulation of toxic vitamin A dimers that may contribute to vision loss.18 A similar clinical trial is being conducted for patients with Stargardt’s and is also expected to be completed in early 2022.18
Clinical take home: Oral supplementation with ALK-001 is in trial for slowing the progression of geographic atrophy in patients with AMD and Stargardt’s.
• LIGHTSITE III
Status: enrolling
Anticipated completion: June 2022
Photobiomodulation (PBM)—a growing trend in medicine—is low-level light therapy that uses specific wavelengths in the visible light to near-infrared ranges, to target certain tissues and stimulate cellular function.19 The idea behind PBM focuses on changes at the mitochondrial level, leading to the upregulation of ATP production, which is a major form of energy necessary for normal cellular functions.19 Other changes produced at the cellular level may also work to reduce oxidative stress.19
LIGHTSITE III is a two-year study consisting of 96 subjects with dry AMD that will receive repeated sham or PBM treatments at several time-points using the Valeda Light Delivery System (LumiThera).20 Findings from LIGHTSITE I demonstrated clinically significant improvements in best-corrected visual acuity and contrast sensitivity after a series of nine treatments over three weeks.19 Approximately 50% of PBM-treated subjects showed improvement of five or more letters vs. 13.6% in sham-treated subjects directly after treatments at one month.19 This was followed by a decline over the next six months and repeated treatments over time will be needed to maintain efficacy.19
Additionally, improvements in drusen volume and thickness were observed, although the authors admitted that more long-term evidence is needed to correlate these anatomical changes with disease regression or progression.19
If this therapy is approved in the United States, optometrists could be at the forefront of providing regular PBM treatments in addition to the close monitoring already provided to patients with dry AMD.
Clinical take home: Photobiomodulation is a low-level light therapy that may improve visual function in patients with dry AMD. Treatments will likely be needed at regular intervals to maintain efficacy.
 |
| ARCHWAY marks a change in anti-VEGF treatment as it focuses on how the therapeutic is delivered, as opposed to the drug itself. Click image to enlarge. |
Wet Macular Degeneration
The most successful area of retina care—anti-VEGF therapy for wet AMD—is swiftly evolving beyond traditional monthly or bimonthly injections to more patient-friendly approaches.
• ARCHWAY
Status: in progress
Anticipated completion: late June 2021
The introduction of intravitreal anti-VEGF agents significantly changed the treatment of wet AMD, allowing patients to retain and even improve visual function. However, repeated injections, often every four to six weeks, place a considerable burden on patients and make it difficult for optometrists to continue follow-up care after referral to ophthalmology.
ARCHWAY is an exciting development in that it studies a new way to deliver anti-VEGF therapy through a port delivery system (PDS, Genentech).21 The PDS is a permanent, refillable intraocular implant that provides continuous delivery of a customized formulation of ranibizumab.22 The device is surgically implanted and then can be refilled in a normal clinical setting.22 ARCHWAY is a Phase III trial that compares PDS refilled every six months to monthly ranibizumab intravitreal injections.21
PDS was shown to be non-inferior and equivalent to monthly ranibizumab injections, both in terms of BCVA and controlling retinal thickness.22 Additionally, 98.4% of patients were able to maintain the six-month refill schedule, which could significantly reduce the number of anti-VEGF treatments to as few as two per year.22 An extension of the ARCHWAY study, called PORTAL, is underway to examine long-term effects of PDS.
At this time, it is difficult to say how this effort might affect optometrists, as refills of the PDS are still necessary to maintain therapeutic levels of anti-VEGF. However, this study marks an exciting change in anti-VEGF treatment, as it focuses on how the therapeutic is delivered, as opposed to the agent itself.
Additionally, there are two trials underway to study the safety and efficacy of PDS in subjects with DME and those with DR without CI-DME, the PAGODA and PAVILION trials, respectively. They are still actively enrolling and are estimated to be completed in September 2024.23,24
Clinical take home: Continuous anti-VEGF therapy through use of an intraocular implant may reduce the frequency of anti-VEGF intravitreal injections in patients with wet AMD and DME.
• OPTIC
Status: in progress
Anticipated completion: June 2022
In one of the most promising trials to date, OPTIC has the potential to greatly reduce the frequency of intravitreal anti-VEGF injections, using gene therapy as a treatment approach.25 The candidate, ADVM-022 (Adverum), is a gene therapy vector that can be delivered in-office by intravitreal injection.25 It contains an adeno-associated virus capsid carrying a coding sequence for aflibercept, which the eye can use to endogenously produce continuous levels of anti-VEGF molecules.26
The safety and efficacy of prolonged intraocular expression of aflibercept was evaluated in preclinical trials and therapeutic levels were confirmed out to at least 30 months with no adverse effects on normal retinal structure or function in non-human primates.26 OPTIC is a Phase I clinical trial studying the safety and tolerability of high and low doses of ADVM-022 in subjects with active choroidal neovascularization secondary to AMD.25
New interim data as of November 2020 showed that ADVM-022 is well tolerated with a favorable safety profile at both high and low doses.27 Secondary outcomes found that mean BCVA is maintained and mean central subfield thickness is maintained or improved at both doses.27 In addition, most patients remain free of supplemental anti-VEGF injections with one patient in the higher dose cohort showing sustained efficacy out to 92 weeks from initial injection.27 In the lower-dose cohorts, two-thirds of patients did not need supplemental anti-VEGF injections with follow-up ranging from 34 to 68 weeks after injection.27
The results of the OPTIC trial thus far are extremely promising and could potentially represent a one-time treatment for wet AMD. This could allow optometrists to continue to be a more active part of a patient’s management and follow-up care, as frequent anti-VEGF injections would be obviated. Two Phase III trials are planned to begin at the end of 2021.28 Additionally, the INFINITY trial, a Phase II study looking at the effect of ADVM-022 on subjects with DME, is set to finish in January 2022.29
Clinical take home: ADVM-022 is a gene therapy vector given as a one-time intravitreal injection and carries a genetic coding sequence for endogenous intraocular production of aflibercept.
 |
| Click table to enlarge. |
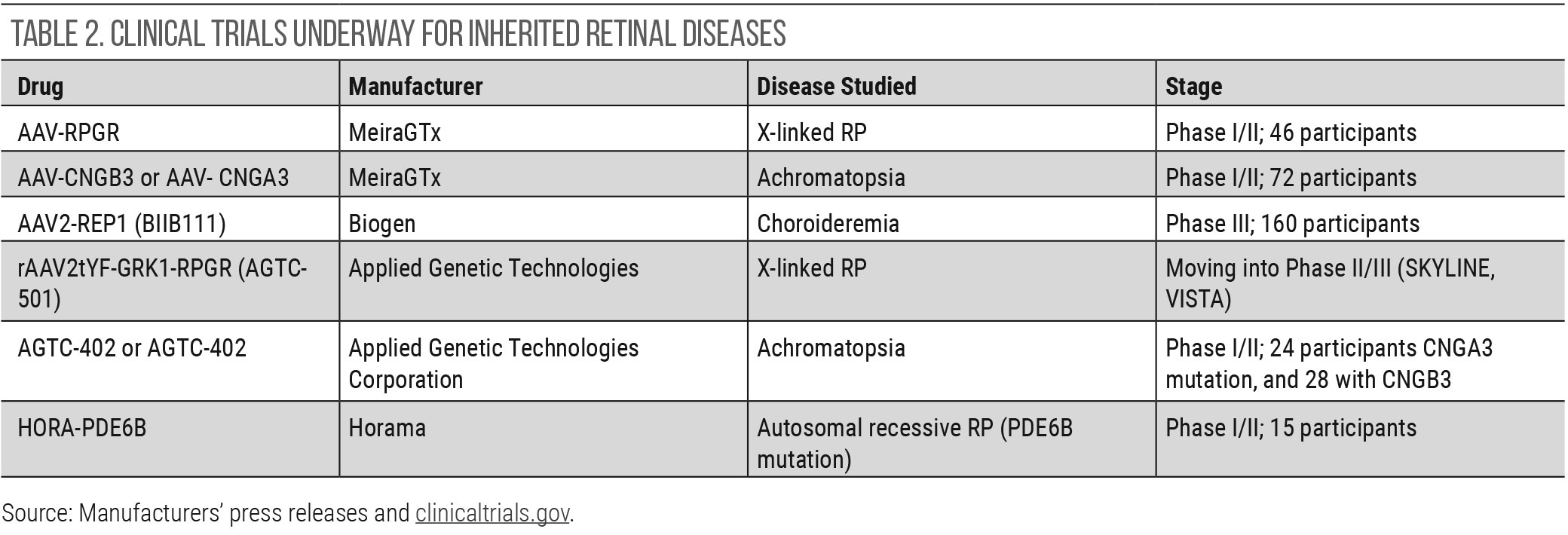
Inherited Retinal Disease
Gene therapy continues to be investigated for inherited retinal diseases (IRDs), even though new applications into wet AMD and DME—described above—have been initiated. At this time, Luxturna (Spark therapeutics) continues to be the only FDA-approved gene therapy for an IRD. It is specific to a biallelic RPE65 mutation that is present in a small subset of those with either Leber’s congenital amaurosis or retinitis pigmentosa.30 Other clinical trials show promise, particularly in choroidermia. Table 2 details the gene therapy trials that are underway for IRDs. The majority of gene therapy trials use an adenovirus vector and subretinal delivery.
Nevertheless, trials with intravitreal delivery and optogenetic gene therapy have also made their way to human clinical trials. However, such a small portion of the population is afflicted with an IRD, which adds to the challenge of designing clinical trials with sufficient participants.
No cost, in-office genetic testing programs for inherited retinal diseases can diagnose these conditions promptly and isolate the causative gene mutation, which helps to identify patients eligible for trials.
Takeaways
At times, the clinical trial landscape in the retina world may seem to be dominated by injectable therapeutics—it certainly has been since the early 2000s—but there are still many retina clinical trials applicable and relevant to optometry. These research projects guide our referral patterns, facilitate our patient education and identify prospective treatment, including some potentially prescribed by ODs. Several of these trials are influencing patient care at the moment, while others should be kept on our radar in the years to come.
Dr. Bedwell is a clinical associate professor at Indiana University School of Optometry. She serves as editor of the Optometric Retina Society’s e-newsletter. Dr. Krenk completed a primary care residency at the Indianapolis Eye Care Center. She is now a visiting lecturer for Indiana University School of Optometry and continues to teach optometry students and see patients at the Indianapolis Eye Care Center. They have no financial interests to disclose.
| 1. ClinicalTrials.gov. Anti-VEGF treatment for prevention of PDR/DME (Protocol W). clinicaltrials.gov/ct2/show/nct02634333. Accessed March 23, 2021. 2. DR Clinical Research Network. Intravitreous anti-VEGF treatment for prevention of vision threatening diabetic retinopathy in eyes at high risk. public.jaeb.org/drcrnet/stdy/340. Accessed March 2, 2021. 3. ClinicalTrials.gov. Study of the efficacy and safety of intravitreal (IVT) aflibercept for the improvement of moderately severe to severe nonproliferative diabetic retinopathy (NPDR)(PANORAMA). clinicaltrials.gov/ct2/show/nct02718326. Accessed March 23, 2021. 4. Eyelea (aflibercept). Highlights of prescribing information. www.accessdata.fda.gov/drugsatfda_docs/label/2019/125387s061lbl.pdf. 5. Puliafito CA, Wykoff CC. New frontiers in retina: highlights of the 2020 angiogenesis, exudation and degeneration symposium. Int J Retina Vitreous. 2020;6:18. 6. DR Clinical Research Network. Randomized trial evaluating fenofibrate for prevention of diabetic retinopathy worsening. public.jaeb.org/drcrnet/stdy/568. Accessed March 30, 2021. 7. ClinicalTrials.gov Fenofibrate for Prevention of DR Worsening (Protocol AF). clinicaltrials.gov/ct2/show/nct04661358. Accessed March 30, 2021. 8. Fenoglide (Fenofibrate) Highlights of prescribing information. www.accessdata.fda.gov/drugsatfda_docs/label/2012/022118s005lbl.pdf. Accessed March 23, 2021. 9. Keech AC, Mitchell P, Summanen PA, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370:1687–97. 10. Chew EY, Davis MD, Danis RP, et al. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: the action to control cardiovascular risk in diabetes (ACCORD) eye study. Ophthalmology. 2014;121:2443-51. 11. ClinicalTrials.gov. Treatment for CI-DME in eyes with very good VA study (Protocol V). clinicaltrials.gov/ct2/show/nct01909791. Accessed March 25, 2021. 12. Baker CW, Glassman AR, Beaulieu WT, et al. DRCR Retina Network. Effect of initial management with aflibercept vs laser photocoagulation vs observation on vision loss among patients with diabetic macular edema involving the center of the macula and good visual acuity: a randomized clinical trial. JAMA. 321(19):1880-94. 13. Roche. (2021, February 12). [Press release]. www.roche.com/media/releases/med-cor-2021-02-12.htm. 14. ClinicalTrials.gov. A study to evaluate the efficacy and safety of faricimab (RO6867461) in participants with diabetic macular edema (YOSEMITE). clinicaltrials.gov/ct2/show/nct03622580. Accessed March 25, 2021. 15. ClinicalTrials.gov. A study to evaluate the efficacy and safety of faricimab (RO6867461) in participants with diabetic macular edema (RHINE). clinicaltrials.gov/ct2/show/nct03622593. Accessed March 25, 2021. 16. ClinicalTrials.gov. Phase III study of ALK-001 in geographic atrophy (SAGA). clinicaltrials.gov/ct2/show/nct03845582. Accessed March 22, 2021. 17. SAGA. www.sagastudy.com/physicians. Accessed March 29, 2021. 18. ClinicalTrials.gov. Phase II tolerability and effects of ALK-001 on Stargardt disease (TEASE). clinicaltrials.gov/ct2/show/nct02402660. Accessed March 22, 2021. 19. Markowitz SN, Devenyi RG, Munk MR, et al. A double-masked, randomized, sham-controlled, single-center study with photobiomodulation for the treatment of dry age-related macular degeneration. Retina. 2020;40(8):1471-1482. 20. ClinicalTrials.gov. Study of photobiomodulation to treat dry age-related macular degeneration (LIGHTSITE III). clinicaltrials.gov/ct2/show/nct04065490. Accessed March 24, 2021. 21. ClinicalTrials.gov. A Phase III study to evaluate the port delivery system with ranibizumab compared with monthly ranibizumab injections in participants with wet age-related macular degeneration (ARCHWAY). clinicaltrials.gov/ct2/show/nct03677934. Accessed March 29, 2021. 22. Genentech. (2020, July 22). [Press release]. gene.com/media/press-releases/14865/2020-07-22/phase-iii-data-show-port-delivery-system. Accessed March 29, 2021. 23. ClinicalTrials.gov. This study will evaluate the efficacy, safety, and pharmacokinetics of the port delivery system with ranibizumab in participants with diabetic macular edema compared with intravitreal ranibizumab (PAGODA). clinicaltrials.gov/ct2/show/NCT04108156. Accessed March 29, 2021. 24. ClinicalTrials.gov. A multicenter, randomized study in participants with diabetic retinopathy without center-involved diabetic macular edema to evaluate the efficacy, safety, and pharmacokinetics of ranibizumab delivered via the port delivery system relative to the comparator arm (PAVILION). clinicaltrials.gov/ct2/show/NCT04503551. Accessed March 29, 2021. 25. ClinicalTrials.gov. ADVM-022 Intravitreal gene therapy for wet AMD (OPTIC). clinicaltrials.gov/ct2/show/nct03748784. Accessed March 29, 2021. 26. Kiss S, Oresic Bender K, Grishanin RN, et al. Long-term safety evaluation of continuous intraocular delivery of Aflibercept by the intravitreal gene therapy candidate ADVM-022 in nonhuman primates. Trans Vis Sci Tech. 2021;10(1):34. 27. Regillo CD. Phase 1 study of intravitreal gene therapy with ADVM-022 for neovascular age-related macular degeneration (OPTIC Trial Cohorts 1–4). Data presented virtually at Angiogenesis 2021 Program. adverum.com/wp-content/uploads/2020/12/november-2020-advm-022-optic-phase-1-data-update.pdf. Accessed March 29, 2021. 28. Adverum Pipeline. adverum.com/pipeline/#wet-amd. Accessed March 29, 2021. 29. ADVM-022 Intravitreal gene therapy for DME (INFINITY). clinicaltrials.gov/ct2/show/nct04418427. Accessed March 29, 2021. 30. Luxturna (voretigene neparvovec-rzyl) Highlights of prescribing information. sparktx.com/luxturna_us_prescribing_information.pdf. Accessed April 1, 2021. |

