Level Up Your TechOptometrists today have more tools than ever at their disposal to help detect, treat and manage disease. To help you keep up with the constant innovation, the September issue of Review of Optometry—our 46th annual technology report—gives readers a rundown on what's new in FAF imaging, visual field testing, ultra-widefield imaging, low vision and more. |
Fundus autofluorescence (FAF) was first described in the 1980s as a means to evaluate and monitor retinal metabolic function. Over time, this testing method has increasingly become important to better understand ocular diseases and the visual function of patients by providing information on the structure and function of the retinal pigment epithelium (RPE).
By exposing the natural fluorophores of the retina to blue or green light, FAF will cause an autofluorescence response that will appear as hyperfluorescence (an increased signal) or hypofluorescence (a decreased signal). This noninvasive imaging method does not require an injection of a dye to take advantage of the autofluorescent qualities of ocular fluorophores to detect early changes and monitor for progression of retinal diseases.1
Retinal Fluorescence
Fluorophores are compounds that absorb light at a certain wavelength and release light in an excited state to become autofluorescent.1 There are several structures of the eye that have fluorophores, including the cornea, lens and retina; however, primarily what will be discussed in this article are those located in the RPE.
The most abundant ocular fluorophore in the retina is lipofuscin. It possesses a mixture of autofluorescent properties that are waste products capable of absorbing blue light at an excitation wavelength of 470nm.1 These waste products are bisretinoid compounds formed in the outer segments of the photoreceptor as byproducts of the visual cycle. They are then deposited in the RPE to be broken down.1 However, in the presence of RPE dysfunction, from conditions such as Stargardt’s disease or age-related macular degeneration (AMD), lipofuscin will accumulate and act as a marker for metabolic activity, providing an early indication for inherited retinal diseases or degeneration of the retina.
 |
|
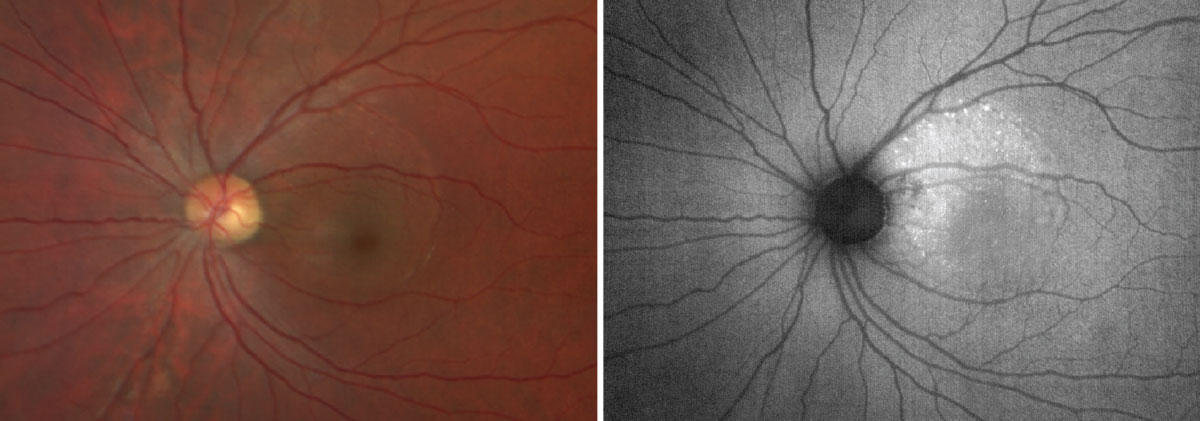
FAF dramatically reveals clinical evidence of central serious chorioretinopathy in this case. Photos: Anna Bedwell, OD, and Brad Sutton, OD. Click image to enlarge. |
Melanin is another ocular fluorophore in the RPE that protects the retina from light-induced damage such as ultraviolet radiation.1 The compound also acts as an antioxidant to protect against free radicals, photo-oxidation and even lipofuscin accumulation. Unlike lipofuscin, melanin has a higher peak of excitation at a longer wavelength of 787nm, so it absorbs the shorter wavelengths of blue (488nm) and green (514nm) light used by FAF and decreases the autofluorescent signal.
Imaging Modalities
Confocal scanning laser ophthalmoscopy (cSLO) uses a system of mirrors to focus a low-powered laser beam to map the retina in a raster-like pattern.1 It uses blue light excitation at a wavelength of 488nm. This generates autofluorescence while at the same time eliminates interference from scattered light due to other ocular structures like the crystalline lens, through confocal optics.1 What’s more, cSLO can capture multiple images and average the quality in real-time to produce high-contrast and high-resolution images. Challenges with using cSLO include a lack of ability to take color photos and the inability to perform testing after fluorescein angiography (FA) since both cSLO and FA use similar excitation wavelengths of 488nm.
 |
|
A normal FAF using blue light. Click image to enlarge. |
Fundus photography is one of the most common imaging modalities due to its simple functionality and cost-effectiveness when compared with other diagnostic devices. The camera uses a single flash of light to capture color photos and autofluorescence through using both blue and green light at an excitation wavelength between 488nm and 585nm, depending on the imaging system and if a red-shifted wavelength filter is used.1 With additional red-shifted wavelength filters, fundus photography can avoid scattered light from crystalline lenses and decrease the absorption of the signal by the natural macular pigments.1 Other advantages of fundus photography include better visualization of exudative retinal diseases, color imaging, enhanced comfort for the patient during testing and that it can still be performed before or after FA.1 Disadvantages of this option include poor image quality if there is interference from media opacities without a red wavelength and a higher chance of pseudo-autofluorescence.
Ultrawide imaging, such as with an Optos, combines confocal scanning and an ellipsoid mirror to create a wide color image and/or FAF photo at up to 200º of view.1 It uses either a 532nm or 633nm wavelength of excitation to capture the autofluorescent properties of the retina, providing a quick capture time, even with an undilated pupil.1 Due to its wide field of view, there is better detection and evaluation for peripheral findings with FAF. The disadvantages include poor views of the superior and inferior retina due to lid and eyelash artifacts, as well as distortion at the peripheral retina due to the ellipsoid mirror.
 |
|
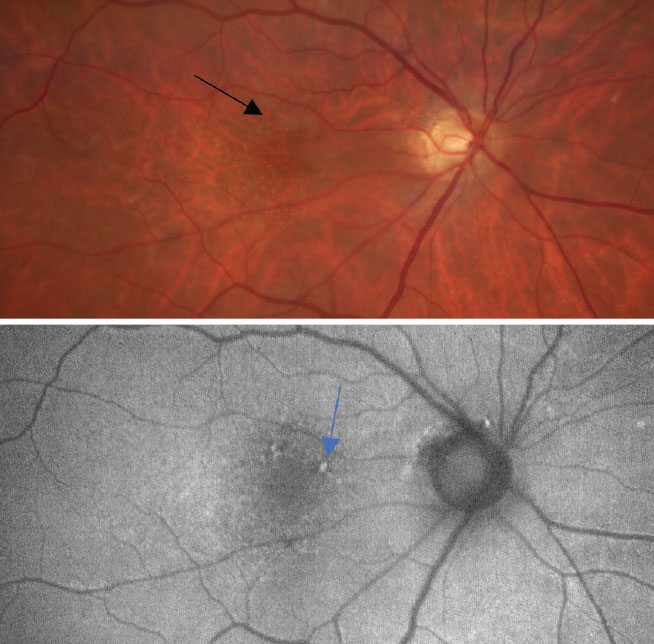
At top, a color photo of early, dry AMD with a few small drusen (<63µm) and mild RPE changes as defined by the Age-Related Eye Disease Study (black arrow). At bottom, an FAF photo that shows small areas of hyperfluorescence and highlights the RPE changes caused by an increase of drusen (blue arrow). Click image to enlarge. |
FAF Interpretation
A healthy FAF should demonstrate a diffuse hyperfluorescent signal because of the natural levels of lipofuscin in the RPE cells, so the posterior pole may appear as a light gray. Depending on the excitation wavelength, the optic nerve will appear as a darker gray when using blue light or a lighter gray when using green light since it lacks RPE or lipofuscin. The fovea will appear as a hypofluorescent spot due to the high amounts of xanthophyll, which naturally absorbs light. Lastly, blood vessels will appear dark, since blood strongly absorbs wavelengths of green and blue light.
FAF and Disease Detection
There are many retinal diseases that can be evaluated with FAF, which in turn can help clinicians better understand a patient’s visual function and predict risk of progression. Below, we will describe a few retinal diseases that may be adequately monitored by using FAF.
AMD. One of the most common causes of permanent vision loss in those over 65 is AMD, accounting for roughly 9% of all causes of blindness worldwide.2 Described as either non-exudative or exudative, this AMD classification depends on the level of changes noted within the RPE and/or the presence of choroidal neovascularization.
Often, many patients during the early stages are asymptomatic but will slowly begin to notice central visual changes as the condition progresses over time. This is especially so in the presence of geographic atrophy as it encroaches into the fovea. In these cases, FAF can be used to map RPE loss and presence of retinal changes that can go unnoticed with funduscopy. An increase in the FAF signal, or hyperfluorescence, marks an area of abnormal metabolic activity from an excess amount of lipofuscin caused by RPE cell death. Conversely, a decrease in the FAF signal, or hypofluorescence, will indicate areas of cellular atrophy or a lack of lipofuscin. It can also be a sign of increased hyperpigmentation of the RPE or new hemorrhaging.
Both autofluorescent qualities can be appreciated in retinal pathologies such as exudative AMD, which can cause an increase in hyperfluorescence from an abnormal amount of lipofuscin as well as leading to areas of hypofluorescence from exudative material and/or hemorrhaging. These can be used to predict the development of a choroidal neovascular membrane.2 However, unlike fluorescein angiography, FAF cannot be used to confirm the presence of a choroidal neovascular membrane.
 |
|
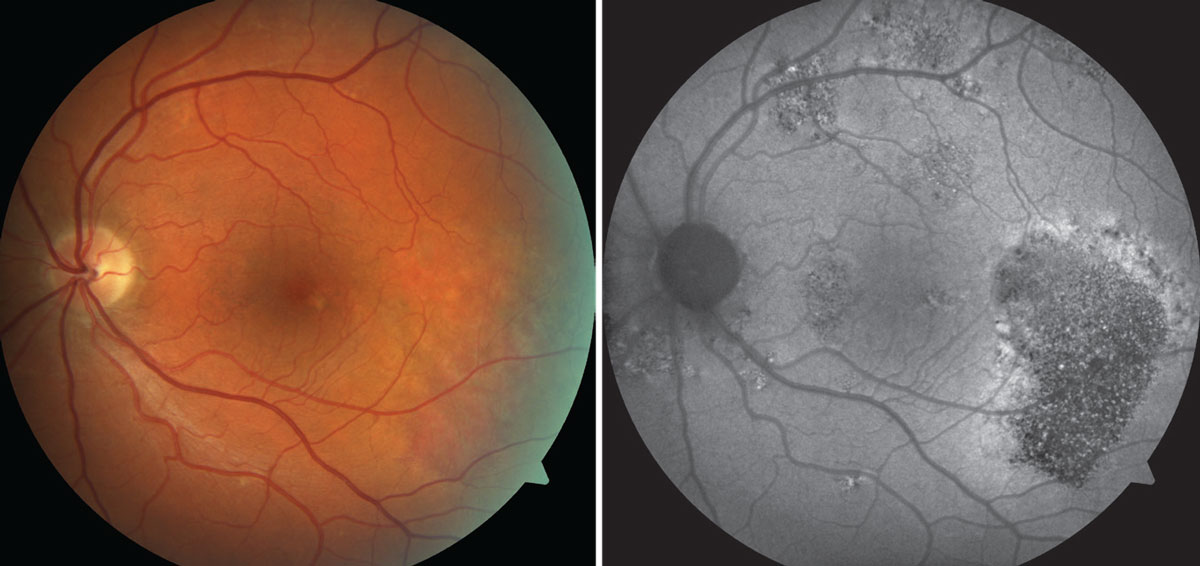
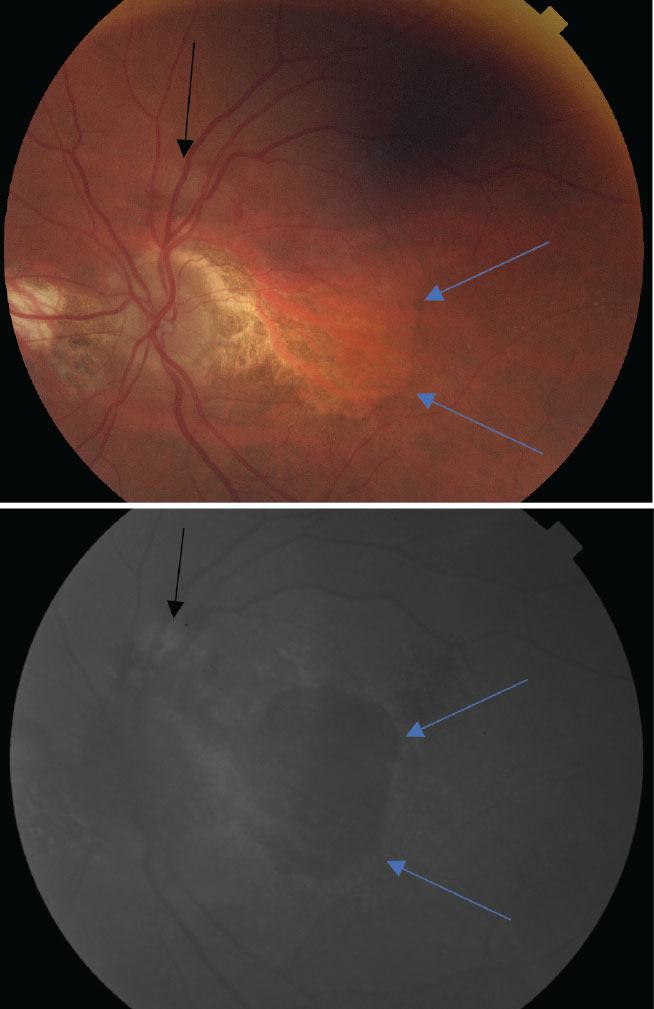
At top, a color photo of advanced AMD with extensive, central geographic atrophy (blue arrows) and a peripheral choroidal neovascular membrane located superiorly to the optic nerve (black arrow). At bottom, an FAF photo that shows an extensive amount of central hypofluorescence due to RPE loss and a small area of hyperfluorescence superior to the optic nerve caused by increased metabolic activity from the neovascular membrane. Click image to enlarge. |
Choroidal lesion. Choroidal melanomas are the most common type of intraocular tumors, but the risk for a nevus to convert to a malignant melanoma is low, at roughly 0.025%.3 Although it is a rare condition, it can become both sight- and life-threatening. This malignancy of choroidal melanocytes leads to the destruction of the RPE and may lead to areas of overlying lipofuscin, atrophy and/or hyperpigmentation.3
There are six main risk factors for tumor growth: thickness (>2mm); subretinal fluid; symptoms of flashes, floaters and vision loss; orange pigment (lipofuscin); acoustic hollowness; and lesion diameter (>5mm).4 In recent studies, FAF has proven to be an essential method for determining the presence of overlying lipofuscin, which relieves the pressure of clinicians having to rely on funduscopic views alone when monitoring for such an important risk factor.
In general, drusen are a hallmark sign for chronicity and increase the likelihood that the lesion is benign in nature, like a choroidal nevus. Presence of lipofuscin, by contrast, is a concern for a malignant lesion, as a lesion causes damage to the overlying RPE, compromising the ability of the RPE to remove excess lipofuscin. This in turn leads to an overlying lipofuscin accumulation. With FAF, both drusen and lipofuscin will cause hyperautofluorescence, but lipofuscin will show a much greater intensity. When evaluating an FAF of malignant lesions, clinicians will notice an overlying mix of autofluorescence, which may appear as a leopard-like pattern. This is caused by multiple focal areas of overlying lipofuscin and increased areas of hyperpigmentation or cellular atrophy from malfunction of the overlying RPE.
Drug toxicity. Hydroxychloroquine is an anti-inflammatory drug that has a risk of causing ocular toxicity. Likelihood of toxicity is increased with a cumulative dosage and long-term usage. Hydroxychloroquine toxicity leads to a loss of parafoveal photoreceptors with foveal sparing and permanent visual changes.1
This presentation will appear as a hyperfluorescent parafoveal ring from photoreceptor damage in the early stages when viewed on FAF. Subsequently, the damage will cause a decrease in FAF signal from RPE cell loss if the medication is not stopped. This late stage is known as bull’s eye maculopathy. Typically, additional testing such as FAF may be used per screening guidelines, since it has a sensitivity of about 73.7% when monitoring for hydroxychloroquine toxicity.1
 |
|
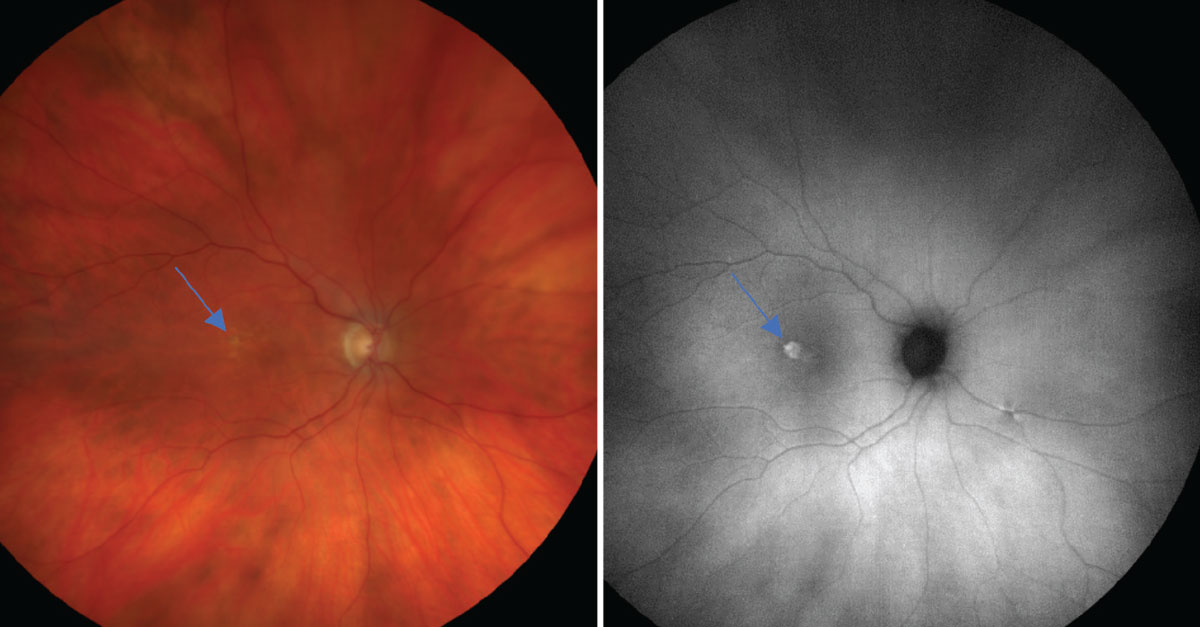
On the left is a color photo of early AMD with a few soft drusen (~63µm), mild RPE changes (as defined by the Age-Related Eye Disease Study) and a new, elevated lesion temporal to the macula (blue arrow). On the right is an FAF photo that shows a significant amount of hyperfluorescence associated at the site of this new lesion, suggesting the presence of a choroidal neovascular membrane from increased metabolic activity. Click image to enlarge. |
 |
|
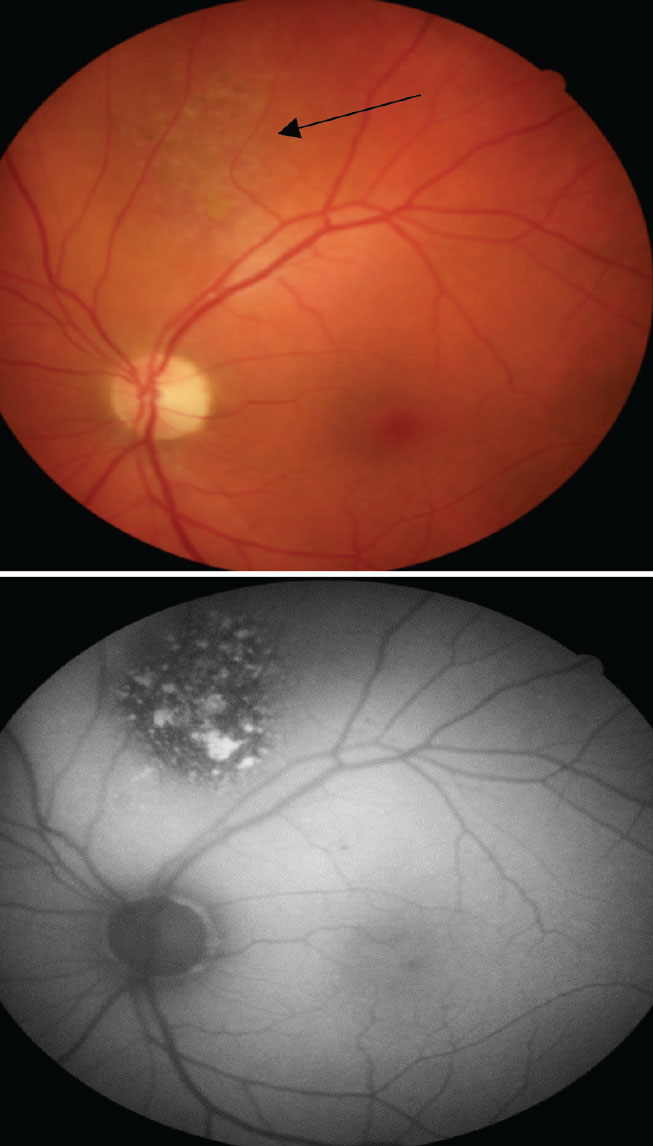
The top color photo reveals a faint, elevated and non-pigmented choroidal lesion (black arrow) that has overlying pigment changes due to RPE changes. On the bottom, the FAF helps to showcase the level of increased metabolic activity with multiple focal areas of hyperfluorescence from an accumulation of lipofuscin and hypofluorescence from RPE atrophy. Click image to enlarge. |
Pattern dystrophies. This collection of late-onset macular dystrophies tend to be bilateral and symmetric. There are different subtypes, including adult-onset vitelliform dystrophy, butterfly pattern dystrophy, reticular dystrophy and fundus pulverulentus. These conditions are associated with an autosomal dominant mutation in the PRPH2 gene, which helps to code for a membrane glycoprotein on cone and rod photoreceptor outer segments.1 This leads to yellow- or gray-like material that accumulates subretinally or at the level of the RPE that gives a hyperfluorescent response on FAF.1
Adult-onset vitelliform dystrophy presents as bilateral subfoveal lesions that consist of outer segment debris because of RPE dysfunction. These vitelliform lesions represent a loss of apposition between photoreceptor tips, and the RPE and will appear egg yolk–like. The lesions cause an increase in the FAF signal during early stages but will become hypofluorescent over time, due to subsequent RPE atrophy.1
Other pattern dystrophies currently do not have as well-documented findings with autofluorescence, but FAF imaging has still been found useful in these conditions. For example, butterfly pattern dystrophy will appear as bilateral pigmented deposits in a butterfly-like shape with central RPE atrophy. When evaluated with FAF, this condition will show areas of hypofluorescence from the deposits and RPE loss causing hyperpigmentation, along with areas of hyperfluorescence caused by increased accumulation of lipofuscin.5 Another pattern dystrophy—reticular dystrophy—will feature a network of pigment lines and knots in a fishnet-like pattern from RPE disruptions, but these lesions will typically fade away with age and lead to atrophic changes. With FAF, there will be increased areas of hypofluorescence from atrophy over time, surrounded by excess lipofuscin-induced areas of hyperfluorescence.5
 |
|
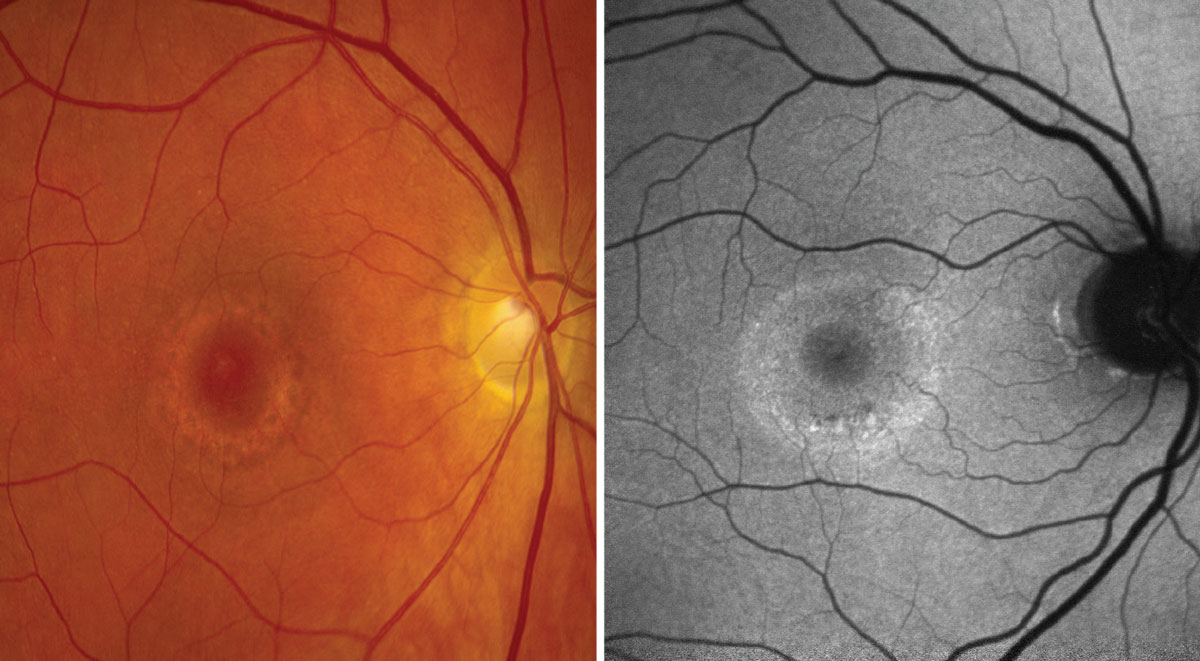
On the left, a color photo showing parafoveal pigmentary changes in a bull’s eye pattern. On the right, FAF shows a large ring of hyperfluorescence surrounding the macula, indicative of active cell death. Click image to enlarge. |
 |
|
On top, a color photo captures a large, central yellow-ish lesion consistent with the appearance of a vitelliform lesion. On the bottom, the FAF photo captures a large area of hyperfluorescence at the site of the lesion from highly concentrated lipofuscin. Click image to enlarge. |
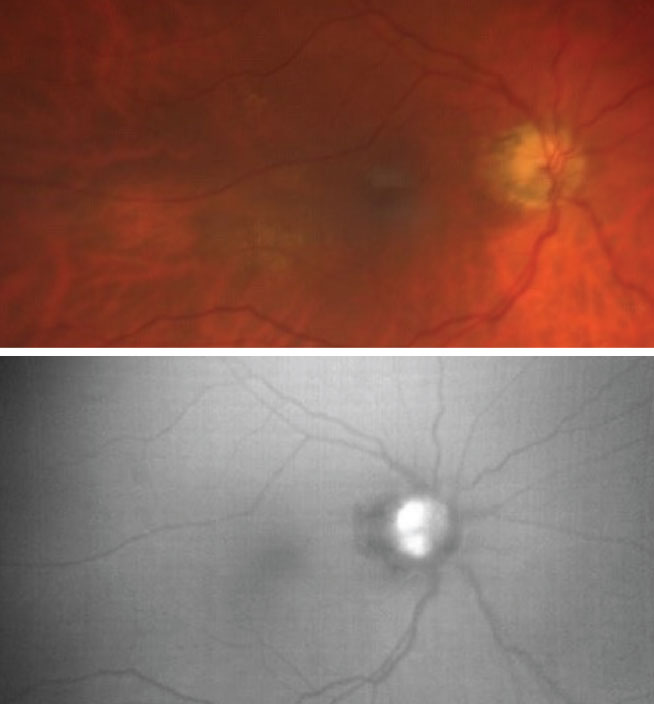
Optic nerve head drusen (ODD). This is a benign and congenital condition of the optic nerve, identified by hyaline-like nodules that may be described as either buried or erupted drusen. These hyaline-like deposits develop because of an abnormal axonal metabolism and continue to change in size over time, hardening with age from calcification.6 As the drusen become more superficial, and thus more visible, they have the ability to cause neural tissue loss that can lead to visual field defects. In cases of buried drusen, FAF is unable to detect the structures due to overlying tissue, but as the drusen become more superficial, they will appear as round or oval-like hyperfluorescent lesions.7 Therefore, FAF can be useful in monitoring for changes in ODD as patients age.
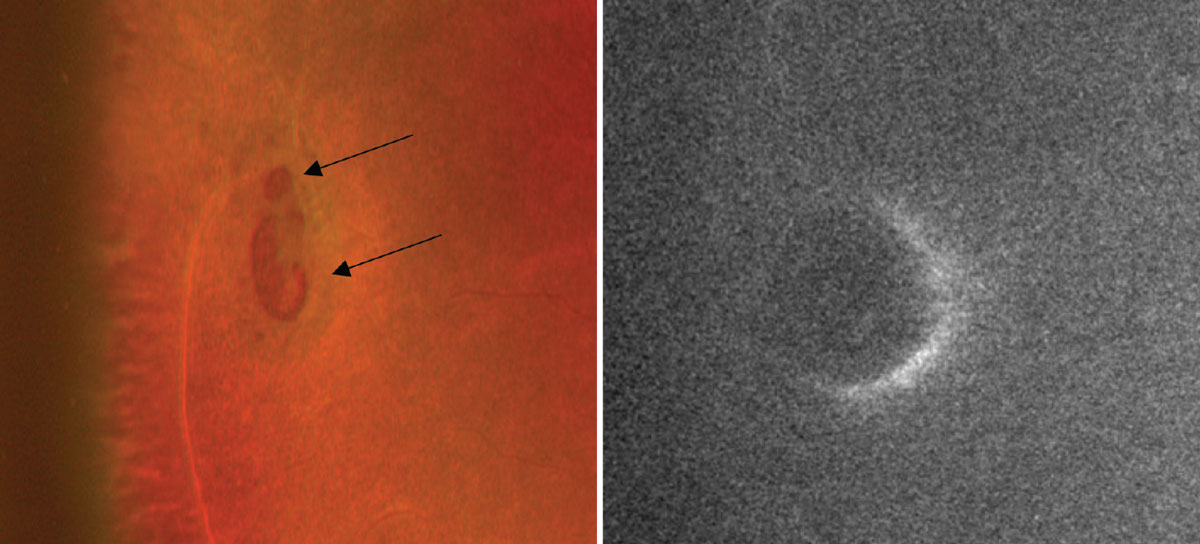
Retinal breaks. Described as holes or tears in the retina, retinal breaks are generally caused by aging or vitreal traction.6 In both scenarios, FAF can monitor for the presence of fluid with retinal breaks, which is a factor considered to increase the risk for a retinal detachment. In cases where patients are asymptomatic but have an atrophic hole, FAF can help through characterizing the presence of a cuff of fluid, which will appear as a hyperfluorescent area near the edges of the hole caused by subretinal fluid. This additional information helps clinicians determine the level of urgency for referrals and prompt treatment with a retina specialist.
Central serous chorioretinopathy (CSCR). This is the fourth most common retinopathy, is caused by choroidal dysfunction and leads to fluid leakage from the RPE into the subretinal space, resulting in serous detachment.1 During initial presentation, roughly 72% to 96% of cases present with an area of hypofluorescence on FAF, which corresponds to the focal leakage sites that would appear on FA.1 As acute cases of CSCR resolve, FAF images will capture granular-like hyperfluorescence from photoreceptor debris, which accumulates in the subretinal space at the border of the serous detachment; this gives the appearance of hyperautofluorescent gravitational tracks. Chronic cases will instead most commonly present a teardrop-like appearance of hypofluorescence due to gravity, marking a sign of atrophy from RPE loss and previous history of subretinal fluid. This is commonly known as hypoautofluorescent atrophic gravitational tracks.1
 |
|
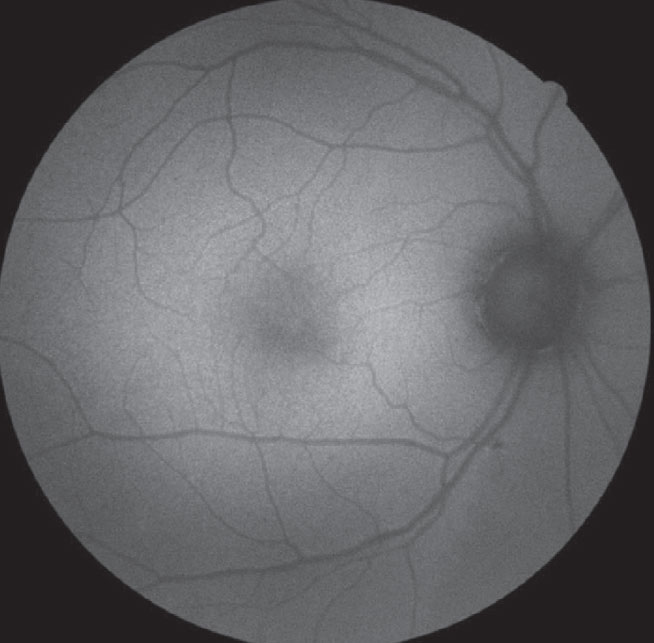
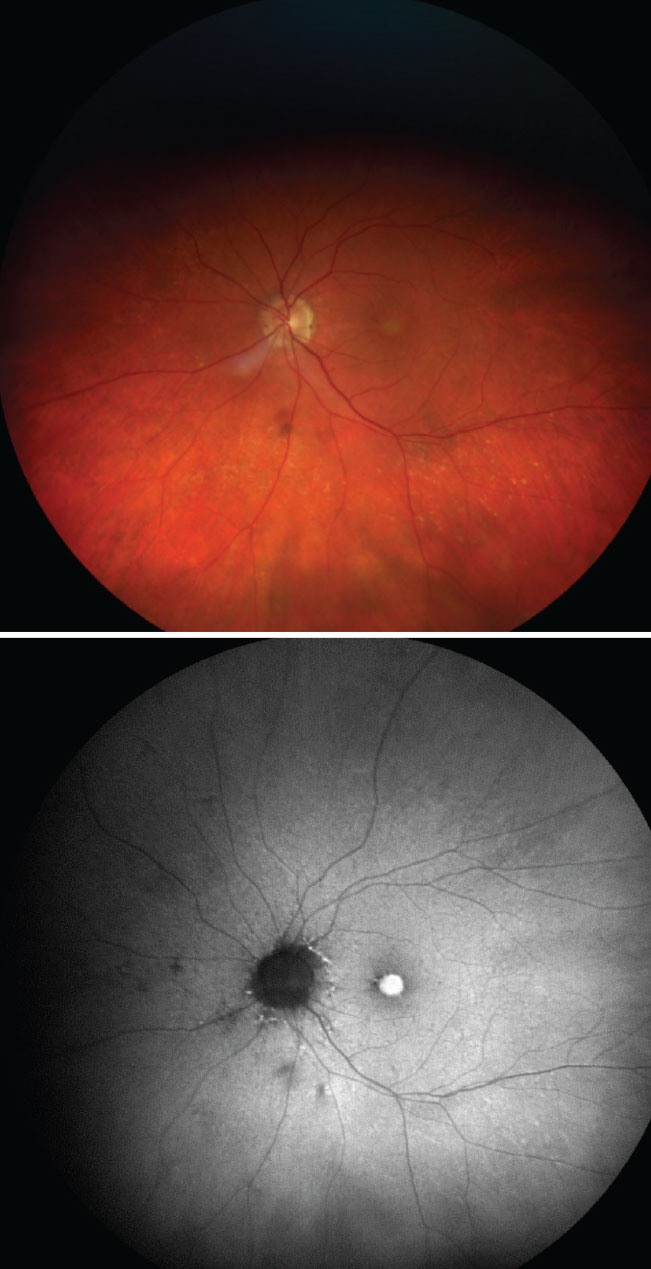
The top color photo captures an optic nerve head with indistinct margins and is suspected for visible ODD. On the bottom, the FAF photo reveals multiple ODD as highlighted by the increased FAF signal overlying the optic nerve head. Click image to enlarge. |
Takeaways
Common etiologies that can lead to an increased FAF signal or hyperfluorescence and subsequent accumulation of fluorophores include an accumulation of lipofuscin, subretinal autofluorescent material (such as vitelliform lesions), ODD and loss of macular photopigment and photoreceptor attenuation.
Hypofluorescence can be just as important on FAF, with common etiologies resulting in RPE loss or blockage causing a decreased FAF signal to occur. These are indicated by a decrease in the natural amount of retinal lipofuscin, the presence of naturally occurring macular pigments (such as xanthophyll), acute retinal hemorrhages, fibrosis or scar tissue and media opacities such as cataracts.
Lastly, FAF has been a valuable method of imaging to characterize the metabolic function of the retina. It is widely available on a variety of diagnostic machines, such as a fundus camera, ultrawide imaging and confocal scanning laser ophthalmoscopy. These options can easily be incorporated into a clinician’s management of retinal pathology. Integrating FAF into your practice strategy can help you detect early changes and monitor for progression with a variety of retinal conditions. This is especially important when regarding preventative care and helping patients to better understand their vision changes as they relate to daily activities such as driving, reading, watching TV and their hobbies. Take that extra step and use FAF on your next eligible patient—you won’t regret it!
 |
||
|
The color photo on the left captures two atrophic holes that are suspected to have a cuff of fluid due to the elevated edges (black arrows). On the right, the FAF photo reveals a crescent-shaped area of hyperfluorescence anterior to the atrophic holes and confirms the presence of a cuff of edema. Click image to enlarge.
|
Dr. Njeru is a staff optometrist at the Chillicothe VA Medical Center. He graduated from the Ohio State University College of Optometry and completed his ocular disease residency at the Chillicothe and Columbus VA. Dr. Grangaard is staff optometrist and serves as the co-residency director at the Chillicothe VA Medical Center. He graduated from the Ohio State University College of Optometry and completed his ocular disease residency at the Chillicothe and Columbus VA. He is a fellow of the American Academy of Optometry and board-certified in medical optometry. They have no financial interests to disclose.
1. Yung M, Klufas MA, Sarraf D. Clinical applications of fundus autofluorescence in retinal disease. Int J Retin Vit. 2016;2:12. 2. Stahl A. The diagnosis and treatment of age-related macular degeneration. Dtsch Arztebl Int. 2020;117(29-30):513-20. 3. Bindewald-Wittich A, Holz FG, Ach T, et al. Fundus autofluorescence imaging in patients with choroidal melanoma. Cancers. 2022;14(7):1809. 4. Shields CL, Dalvin LA, Ancona-Lezama D, et al. Choroidal nevus imaging features in 3,806 cases and risk factors for transformation into melanoma in 2,355 cases: the 2020 Taylor R. Smith and Victor T. Curtin Lecture. Retina. 2019;39(10):1840-51. 5. Ahmed H, Sierpina DI, Khazaeni L. Retinal pattern dystrophy. In: Statpearls [Internet]. Treasure Island (Fl): StatPearls Publishing. Updated January 7, 2023. 6. Friedman NJ, Kaiser PK, Pineda II R. The Massachusetts Eye and Ear Infirmary illustrated manual of ophthalmology. 5th ed. St. Louis, MO: Elsevier. 2021. 7. Tuğcu B, Özdemir H. Imaging methods in the diagnosis of optic disc drusen. Turk J Ophthalmol. 2016;46(5):232-6. |