In April 2016, optometry lost a giant when the author of the seminal work Primary Care of the Posterior Segment, Larry Alexander, OD, died. In addition to being a physician, author and educator at the University of Alabama Birmingham School of Optometry, Dr. Alexander was a past president of the Optometric Retina Society (ORS). That group has chosen to honor his legacy by accepting case reports from optometric residents across the country relating to vitreoretinal disease for publication in Review of Optometry. As selected by the board of the ORS, this case won the first Larry Alexander Resident Case Report Contest.
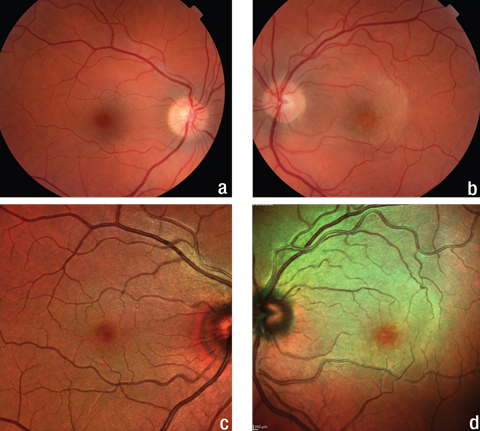 |
| Fig. 1. The fundus and multicolor images of the patient’s right eye (a, c) show no abnormalities, but the same images of her left eye (b, d) show a yellow, plaque-like lesion extending from the optic nerve into the superior macula. Click image to enlarge. |
In March 2016, the Centers for Disease Control and Prevention (CDC) issued a clinical advisory for ocular syphilis due to a 12-case cluster reported in San Francisco and Seattle over four months.1 Although ocular involvement from syphilis is rare, increasing incidence rates suggest clinicians must consider syphilis on their list of differential diagnoses.
This article describes a rare case of acute syphilitic posterior placoid chorioretinitis (ASPPC) in an immunocompetent female secondary to syphilis. It also provides a brief review of systemic syphilis and an in-depth review of the literature regarding ASPPC, as well as discusses the laboratory evaluation and treatment of confirmed systemic syphilis.
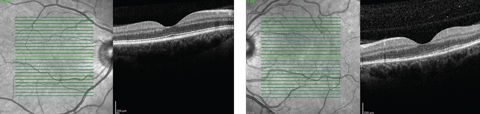 |
| Fig. 2. While our patient’s right macula appeared normal on OCT, her left eye demonstrated a disruption of the PIL. Click image to enlarge. |
Back in the States
A 46-year-old female presented to the clinic with concerns of a black spot in the central vision of her left eye. She reported the spot appeared upon awakening the day before and had gotten worse. The patient’s ocular history was unremarkable. Her last eye exam was one year prior at a different clinic. She had recently noted swollen lymph nodes on her abdomen after a trip to Mexico. Further imaging showed cysts on her ovary near the swollen lymph nodes and she was scheduled for an MRI the next week. She acknowledged high levels of stress secondary to the recent death of her best friend, but denied recent illness and neurologic symptoms.
Her medical history was significant for post-traumatic stress disorder, anxiety, chronic neck pain, gestational diabetes and gastroesophageal reflux disease. Her medications included methocarbamol, omeprazole, oxybutynin chloride and venlafaxine.
Syphilis BasicsSyphilis is a sexually transmitted systemic infection caused by the spirochete bacterium Treponema pallidum. Despite the common perception that syphilis is a disease of the past, the CDC reports that 2015 saw the highest rate of cases of primary and secondary syphilis since 1994.2,3 Ocular involvement in syphilis—though rare—is notoriously difficult to diagnose, due to the disease’s ability to affect almost any part of the eye and mimic other inflammatory disorders.4-7 In a minority of patients with ocular syphilis, a distinctive oval, yellow, plaque-like chorioretinitis in the outer retina of the macular or juxtapapillary areas develops. This disease entity—acute syphilitic posterior placoid chorioretinitis— is estimated to occur in only 3% of patients with ocular syphilis.6-8 A study in 2012 reported only 60 known cases of ASPPC, of which only 13% occurred in females.7 |
Diagnostic Data
Her entering visual acuities (VA) were 20/20 OD and 20/70+2 OS with no improvement on pinhole. Extraocular motilities, confrontation visual fields and pupils were all normal. During pupil testing, the patient noted the light was dimmer in the left eye than the right. Amsler grid testing showed a large central scotoma in her left eye.
Slit lamp examination of the anterior segment was normal with quiet anterior chambers in both eyes. Intraocular pressures (IOP) were normal. There was trace vitreous cell in the right eye and grade one vitreous cell in the left. Retinal evaluation revealed a normal retina in the right eye and a yellow plaque-like lesion in the outer retina of her left eye extending superiorly from the nerve (Figure 1).
Macular optical coherence tomography (OCT) revealed a normal foveal contour with significant disruption of the photoreceptor integrity line below the fovea and extending through the superior nasal macula in the left eye (Figure 2). Fundus autofluorescence (FAF) demonstrated hyperautofluorescence around the optic disc and superior macula in the patient’s left eye (Figure 3).
Due to the patient’s age and the acute nature of the vision loss, we recommend she undergo fluorescein angiography (FA) that day for further evaluation. The FA showed early mild hypofluorescence around the optic disc and superior macula with mottled hyperfluorescence in the area of the plaque in the late phase in the left eye (Figure 4).
Based upon the FAF, FA and OCT results, the retinal specialist who completed the FA was concerned about the unknown etiology of the posterior uveitis.
We ordered a comprehensive lab work-up, which included a complete blood count, a comprehensive metabolic panel, syphilis serologies, Quantiferon test for tuberculosis, toxoplasmosis serology and toxocariasis serology and a chest x-ray. A follow-up was scheduled in three days.
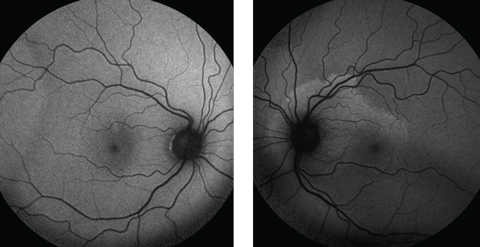 |
| Fig. 3. Fundus autofluorescence demonstrates a normal right fundus, but the left shows mottled hyperfluorescence around the optic nerve and superior macula, indicating lipofuscin accumulation in the RPE. Click image to enlarge. |
Diagnosis
At follow-up, the patient reported improved vision and a decrease in the size of the black spot. Uncorrected VA was 20/20 OD and 20/30 OS. Entrance testing, IOP and anterior segment evaluation were normal in both eyes. No change was seen in the appearance of either fundus. OCT of the left macula showed outer retinal disturbances with new hyperreflective areas at the RPE layer and extension of these changes into the inferior macula since the previous scan (Figure 5). FAF imaging revealed enlargement of the hyperfluorescent area (Figure 6).
All of the lab tests ordered at the initial presentation were normal, except for a positive enzyme immunoassay (EIA) used to assess for syphilis. Confirmatory testing for syphilis with the rapid plasma reagin (RPR) and T. pallidum particle agglutination assay tests were positive, and the RPR test showed a titer of 1:512. HIV titers, as well as screenings for gonorrhea and chlamydia, were all negative.
Based on the clinical and laboratory findings, we diagnosed the patient with ASPPC. At this point the patient revealed a history of unprotected intercourse with a promiscuous sexual partner who had a small, round, painless lesion on his genitals for the past month. She also reported several painful ulcers on her tongue and inside of her lower lip and a sore throat.
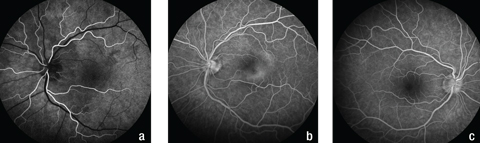 |
| Fig. 4. Fluorescein angiography shows (a) hypofluorescence superior to the optic disc and around the left macula in the early phase, (b) mottled hyperfluorescence, known as “leopard spotting,” in the area of the plaque lesion in the late phase in the patient’s left eye and (c) normal fundus appearance during the late phase in the right eye. Click image to enlarge. |
Treatment
The patient was referred to an infectious disease specialist, who completed a lumbar puncture was completed. A Venereal Disease Research Laboratory (VDRL) test on her cerebrospinal fluid (CSF) was negative. Although the standard treatment for syphilis is penicillin, the patient had a significant penicillin allergy and reported a blistering rash, mouth sores and difficulty breathing within 30 minutes of taking the drug on two separate occasions. Therefore, she was started on two grams of intravenous (IV) ceftriaxone per day for 14 days.
The patient was kept in the hospital for her first dose due to the small risk of cross-reactivity between penicillin and cephalosporin drugs. She reported throat itching and pain during the initial dose, but there were no objective signs of allergic reaction and the two-week treatment was completed at home.
At her six week follow-up, the patient felt her vision had improved further. VA was 20/20 OD and OS. Retinal examination showed mild RPE mottling in the macula OS with resolution of the yellow-plaque lesion. OCT showed resolved PIL disruption subfoveally, with remaining mild outer retinal granularity in the inferior macula and FAF imaging was normal (Figures 5 and 6). Despite the mild vitreous cell in her right eye at the initial exam, there were no signs of further ocular involvement.
Table 1. Differential Diagnosis of the Posterior Uveitides7,26-28 | |||
| Cause | Clinical Setting | Ancillary diagnostic testing | |
| Ocular | Systemic | ||
| Idiopathic | Diverse | Diagnosis of exclusion | |
| Infectious | |||
| Toxoplasmosis | Patch of white retinochoroiditis with speckled borders next to a pigmented scar | FA: hypofluorescence in the area of an old chorioretinal scar, early hypofluorescence and late hyperfluorescence with persistent leakage during active infection | Serum antibodies; High frequency false positives |
| CMV | Immunosuppressed patient with broad white patches of retinitis, often associated with hemorrhages | None | None required; HIV screening if no current diagnosis |
| Toxocariasis | Child with a white, round vitreous/inner retinal mass often extending from the optic nerve | None | Serum ELISA for anti-Toxocara antibodies |
| Tuberculosis | One or multiple slightly raised yellow, deep retinal patches with indistinct borders | None | PPD or Quantiferon gold |
| Syphilis | Any posterior uveitis | Varies depending upon presentation | VDRL, RPR, FTA-ABS |
| ARN | Patches of peripheral, white retinal necrosis | FA: peripheral areas of retinal arteriolar capillary nonperfusion | Aqueous PCR for VZV or HSV DNA |
| PORN | Immunosuppressed patient with deep, multifocal retinal lesions sparing retinal vasculature; often bilateral | FA: confirms lack of vasculitis | Aqueous PCR for VZV or HSV DNA |
| Inflammatory | |||
| Sarcoidosis | Yellow-orange deep fundus lesions or larger peripheral full thickness white nodules; sometimes accompanied by an exudative vasculitis | FA: hypofluorescence in area of ischemia, venular leakage in area of inflamed vessels | ACE, chest x-ray |
| POHS | Classic triad of peripapillary atrophy, mid-peripheral histo spots, and macular lesions; vitreous cell will be absent | None | None |
| APMPPE | Creamy yellow deep retinal patches with indistinct borders | FA: early hypofluorescence with late hyperfluorescence in the area of active disease; ICGA: perfusion of the large choroidal vessels in the area of hypofluorescence in the early phase and marked choroidal hypofluorescence in the late phase | None |
| Serpiginous choroidopathy | Bilateral, well-circumscribed patches of yellow choroiditis that begin near the disc and extend in any direction | FA: early hypofluorescence with later hyperfluorescence in the area of active disease; hyperfluorescence begins at the margins of the lesion and spreads inward; ICGA: hypofluorescence through early and late phases; may be areas of hyperfluorescence outside the clinical lesion | None |
| Birdshot retinochoroidopathy | Multiple white/yellow oval spots with indistinct borders; often mostly nasally | FA: lesions aren’t visible until late in the disease process, when they stain; ICGA: late phase hypofluorescence. Detects a greater number of spots than seen clinically; ERG: delayed implicit time and b-wave amplitude | HLA-A29 |
| PIC | Few clustered white spots with distinct borders, no vitritis, usually women | FA: punctate hyperfluorescent late staining | None |
| MEWDS | Multiple subtle, deep, gray spots | FA: early hyperfluorescence that persists into late phase; ICGA: hypofluorescence in the area of the white dots, extensive area of hypofluorescence surrounding the optic disc; VF: enlarged blind spot; ERG: depressed A-wave, recovers during resolution of the disease | None |
| VKH Syndrome | Nonrhegmatogenous retinal detachments, often with deep, gray choroidal spots; patients often note tinnitus or severe headaches | FA: diffuse, pinpoint hyperfluorescent leaks at the level of the RPE, late pooling in the subretinal space; ICGA: multiple hypofluorescent spots; Ultrasound: shifting exudative RD | Often involves multi-system work-up including LP, audiogram, pathology, and genetic testing for HLA-DR405 |
| CMV: cytomegalovirus, ARN: acute retinal necrosis, PORN: progressive outer retinal necrosis, POHS: presumed ocular histoplasmosis syndrome, APMPPE: acute posterior multifocal placoid pigment epitheliopathy, PIC: punctate inner choroidopathy, MEWDS: multiple evanescent white dot syndrome, VKH: Voyt-Koyanagi-Harada. | |||
Discussion
In cases of acquired syphilis, transmission of T. pallidum occurs through direct contact with an infectious lesion during sex. Four stages of disease have traditionally been described: primary, secondary, latent and tertiary. The primary stage is characterized by a painless lesion, known as a chancre, at the site of inoculation after an average incubation period of 21 days.5,9 The secondary stage begins a few weeks to months after the chancre develops in 25% of untreated patients and is associated with a wide range of symptoms.10 Latency may follow the secondary stage. The patient is asymptomatic during latency, and may remain in latency indefinitely, relapse into the secondary stage or develop tertiary syphilis. Tertiary syphilis can occur from one to 30 years after primary infection and is when the most serious systemic manifestations occur.5,9
Neurosyphilis is a distinct category of the disease that can occur at any time during the course of infection, and is regarded as any central nervous system manifestation. Distinction of neurosyphilis is important due to unique diagnostic and treatment guidelines.11 Eye care providers should be aware that any ocular involvement is considered a manifestation of neurosyphilis.
Ocular involvement in syphilis most frequently occurs during the secondary and tertiary stages of the infection, but has been reported in all stages.4-7 On average, 1% to 2% of cases of ocular inflammation are estimated to be secondary to syphilis, and the ocular manifestations are extremely varied.5,12 Only 3% of cases of ocular syphilis result in the distinctive oval, plaque-like chorioretinitis known as ASPPC.
ASPPC has been described as bilateral and unilateral with a mean age of 40 at presentation.7 ASPPC is significantly more rare in women than men (13% vs. 87%).7 Presenting visual acuity ranges from 20/20 to count fingers, with a median of 20/80.7 Mucocutaneous manifestations are the most common associated systemic finding with ASPPC, while anterior chamber or vitreous inflammation are the most common concurrent ocular findings.6,7,13-16
 |
 |
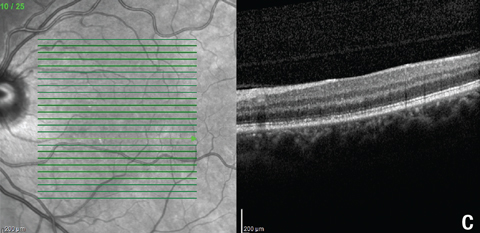 |
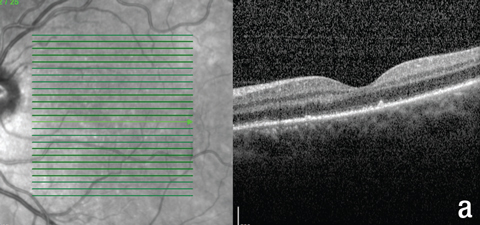
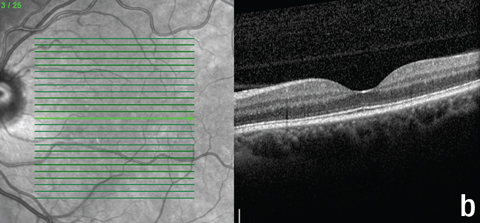
| Fig. 5. These OCT images show the patient’s macula, (a) at the first follow-up, and (b) and (c) at the second follow-up. Note the new hyperreflective granular area in (a), resolution of the subfoveal PIL disruption in (b), and residual outer retinal granularity in the inferior macula in (c). |
Identification
ASPPC has distinct clinical, angiographic, autofluorescence, multifocal ERG (mfERG) and OCT features. On funduscopic exam, all eyes with ASPPC exhibit a yellow, circular or oval placoid lesion in the outer retina, rarely extending beyond the posterior pole.6 FA shows early hypofluorescence within the lesion followed by progressive punctate hyperfluorescence, known as “leopard spotting,” in the late stage.6,7 Although FA has been used more widely for evaluation of ASPPC, some authors suggest indocyanine green angiography (ICGA) may be better suited for evaluation to detect the full extent of choroidal inflammation.25
FAF is gaining popularity in eye care due to its ability to provide a qualitative measure of the structure and function of the RPE.25 FAF imaging during the acute stage of ASPPC shows punctate hyperautofluorescent spots within the lesion, consistent with lipofuscin accumulation at the RPE-photoreceptor junction.6,7 Resolution of hyperautofluorescence on FAF, indicating improved RPE functioning, correlates to improvement in visual acuity.25 This correlation suggests FAF may be a useful noninvasive tool for monitoring progressive inflammatory disorders such as ASPPC.25
OCT findings in the acute phase of ASPPC show thickening and hyperreflective nodularity of the RPE with disruption of the overlying photoreceptor integrity line (PIL).18 In a minority of cases, OCT has revealed subretinal fluid.23,24 Post-treatment, OCT imaging shows resolution of the RPE nodules with remaining mild disruption of the PIL.18 Just as with FAF, return of the PIL on OCT correlates to improvement of visual acuity.
One study used serial mfERGs throughout the course of ASPPC in a single patient.23 Their findings showed a reduced mfERG during the acute phase of the disease followed by a delay in the improvement of the mfERG compared with the improvement in visual acuity.23 The authors concluded that their results suggest full photoreceptor recovery may actually be delayed compared with the apparent anatomical recovery on OCT.23
Good visual outcome after ASPPC is possible with and without prompt antibiotic therapy.6,7,16,20 One study acheived improvement in VA to 20/25 or better in approximately 90% of cases.6,7,15,16 Decreased vision after treatment was associated with persistent, significant disruption of the outer retinal layers, although mild disruption of these layers may remain in patients whose acuity returns to baseline.6,23
Differential diagnoses for ASPPC should include most posterior uveitides (Table 1).7,26-28 Differential diagnosis of the posterior uveitides is often possible based upon history, examination and imaging (particularly FA, FAF and ICGA).25,28
Although resolution of ASPPC has been documented without treatment, appropriate diagnosis and treatment is critical to prevent subsequent systemic or ocular manifestations. Imaging tools can help raise suspicion of ASPPC, but accurate diagnosis of syphilis requires laboratory testing. Lab testing for syphilis is notoriously complex due to multiple testing algorithms and myriad tests available. All lab tests can be divided into treponemal specific vs. non-treponemal specific. Tests from both categories must be used to make a diagnosis.
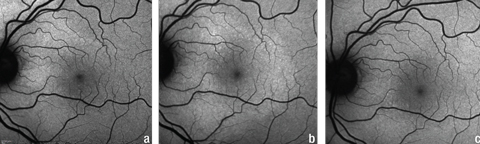 |
| Fig. 6. Serial FAF photos of the left eye at (a) initial presentation, (b) first follow-up, and (c) second follow-up. Note the extension of the hyperfluorescence inferior to the optic nerve and macula in (b) and resolution of hyperfluorescence in (c). These findings were consistent with the changes in the PIL shown in Figure 5. Click image to enlarge. |
Testing
Non-treponemal tests assess the reactivity of serum to a cardiolipin-cholesterol-lecithin antigen and are, therefore, nonspecific for syphilis.3 Traditionally, these tests were used for initial screenings, due to their low cost, ease of performance and quantifiability.3 Results from these tests are given in a titer, which gives an indirect indication of activity of the infection and allows treatment response to be monitored. Our patient had a titer of 1:512, meaning her serum could be diluted 512 times and still be reactive.
Treponemal specific tests are traditionally used as confirmatory tests after a positive non-treponemal test because of their higher complexity and cost.3 These tests are specific for serum antibodies against treponemal antigens, but are qualitative only. Newer, more automated versions of treponemal tests now exist, and are increasingly being used as the initial screening rather than a non-treponemal test.29 These modern tests have flipped the traditional testing algorithm on its head, but positive tests from both the non-treponemal and treponemal categories are still required for diagnosis. New point-of-care tests that detect both treponemal and non-treponemal antibodies may become the future of syphilis diagnostic testing.30
All patients with suspected neurosyphilis require examination of the CSF to make a definitive diagnosis, and should also undergo testing for HIV.2,3,31,32 A positive CSF-VDRL is highly specific for neurosyphilis, but sensitivity is very poor. The test may be negative in up to 70% of people with neurosyphilis, as was the case in our patient.32 Of the patients in the case studies of ASPPC reviewed, only 50% to 75% had positive CSF testing.6,7 Regardless of the results of CSF testing, patients with ocular involvement should be treated according to neurosyphilis guidelines. If CSF testing is positive, treatment response should be monitored via CSF rather than serum testing.
Medical Intervention
Penicillin has been the treatment of choice for syphilis since its introduction in the mid-1900s. Prolonged levels of penicillin are required to eliminate T. pallidum, and the route of administration and duration varies depending on the stage of the disease. In cases of neurosyphilis due to poor penetration of the ocular structures and central nervous system via IM administration, IV penicillin G is given.33 Adequate treatment response is considered a fourfold decline in the non-treponemal titer.7 Most patients remain serofast after treatment with stable titers for life.33,34
Treatment of patients who are severely allergic to penicillin involves an alternative agent such as the tetracyclines or ceftriaxone. There is limited data to support the dosage, duration and efficacy of these alternatives.3,33 This was the case in our patient, and treatment with IV ceftriaxone resulted in a good clinical response.
ASPPC is a rare manifestation of syphilis with distinctive clinical and angiographic features. The disease seems to follow a course of onset-aggravation-resolution, sometimes prior to treatment. The pathophysiology of ASPPC is not well understood, but recent studies of FAF and mfERG findings indicate choroidal and RPE inflammation with significant, often long-lasting, disruption of the photoreceptors.
Because the CDC has issued a clinical advisory for ocular syphilis, more patients may be presenting with manifestations of the disease. Since only 50% of patients with ocular syphilis have any systemic signs, eye care providers must maintain a high index of clinical suspicion to make a diagnosis. Syphilis is much more likely to present in men, but as shown in this report, women may also be affected. Patient history, clinical examination, imaging characteristics and targeted laboratory testing are critical for diagnosis in cases of posterior uveitis of unknown etiology. All patients diagnosed with ocular syphilis should be tested for both neurosyphilis and HIV coinfection. In addition, comanagement with an infectious disease specialist is appropriate to ensure proper treatment and fulfillment of reporting and counseling requirements set forth by the CDC.
Dr. Dailey practices in the Optometry Department of the Veteran’s Administration Puget Sound Health Care System – American Lake. She is the winner of the First Annual Larry Alexander Resident Case Report Contest. She would like to acknowledge Jeffrey Hiett, OD, and Judith Oh, OD, for their guidance and expertise during the writing process.
1. Centers for Disease Control and Prevention. Clinical Advisory: ocular syphilis in the United States 2015. Atlanta, GA: US Health and Human Services, CDC; 2016. http://cdc.gov/std/syphilis/clinicaladvisoryos2015.htm. Accessed January 8, 2017. |

