 |
Oncology has seen tremendous advancements in a few short years, including multiple FDA approvals in a rapidly expanding class of targeted therapies known as antibody-drug conjugates, or ADCs. These cancer-targeting “smart bombs” consist of a monoclonal antibody bound to an anti-cancer payload using a chemical linker. While the tolerability of ADCs is generally favorable compared to conventional chemotherapy, several ADCs are associated with adverse ocular surface reactions that are managed by adjusting the medication schedule and dosage.1 These patients are often seen in optometry offices because the ocular signs indicate an adjustment in dosing concentration is required.
Elahere (mirvetuximab soravtansine-gynx), a new targeted cancer therapy, has been shown to improve overall survival in patients with recurrent ovarian cancer, and optometrists can help significantly.
Elahere and Ovarian Cancer
Elahere is the third ADC to launch with an eyecare management plan that requires patients to undergo monitoring by an eyecare professional while on the medication.1 Ovarian cancer is a difficult-to-treat malignancy, with most patients presenting with late-stage disease and typically will undergo surgery followed by platinum-based chemotherapy. Unfortunately, about 80% experience recurrence and go on to develop platinum-resistant ovarian cancer.2,3 The monitoring for Elahere falls well within the expertise of optometry, allowing us to help oncologists respond to the characteristic corneal epithelial changes, and in doing so, help patients receive the full benefit of their cancer therapy by enabling intervention before drug discontinuation becomes necessary.
The MIRASOL confirmatory trial, designed to support conversion to full approval, demonstrated clinically significant improvements in key efficacy endpoints (progression-free survival, objective response rate and overall survival) compared to standard-of-care chemotherapy, making Elahere the first novel therapy to show an overall survival benefit in recurrent ovarian cancer.4 The most common adverse reactions were low-grade gastrointestinal, neurosensory and a keratopathy known as microcyst-like epithelial changes (MECs).2
 |
|
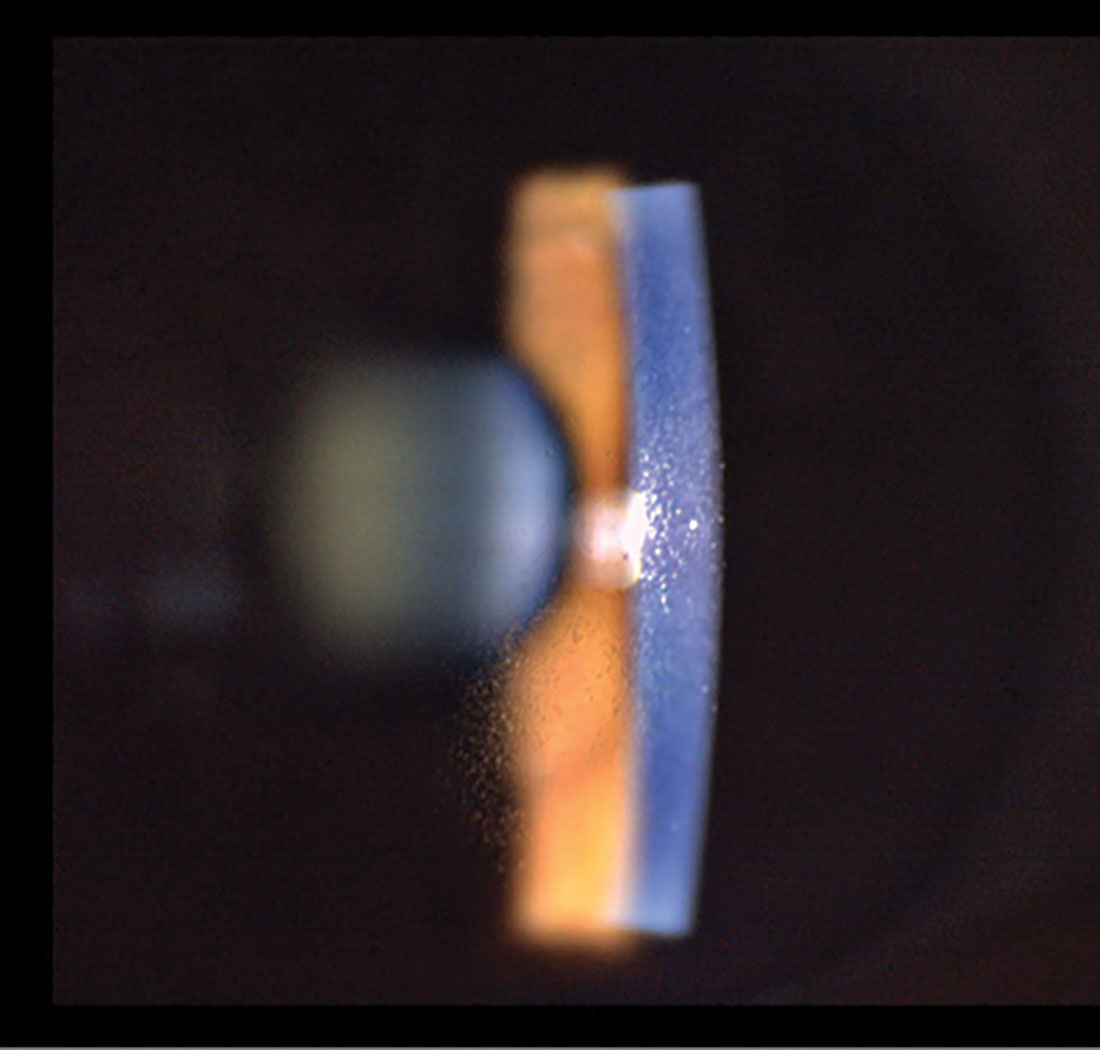
Paracentral non-confluent MECs. Click image to enlarge. |
MECs and Dry Eye Symptoms
In a pooled safety analysis of 464 patients treated across three clinical trials of Elahere, this keratopathy was observed in 36% of patients. It was generally characterized by MECs with or without punctate epithelial erosions. Furthermore, 26% of the patients reported dry eye sensation.1
On slit lamp exam, MECs appear as fine, non-staining punctate epithelial opacities. On retroillumination, MECs may take on a water droplet–like appearance (Figure 1). They may be numerous and are often observed in a circumferential pattern in the mid-peripheral epithelium with or without central involvement.1,5,6
MECs may cause changes in vision, as half of affected patients reported impairment, which included blurred vision associated with changes in refraction and temporary reductions in visual acuity.2 Published case reports show an association between the location of MECs, refractive shifts and changes in corneal topography that are hypothesized to result from transient changes in the corneal epithelial thickness profile.6,7
Mitigation, Management and Modifications
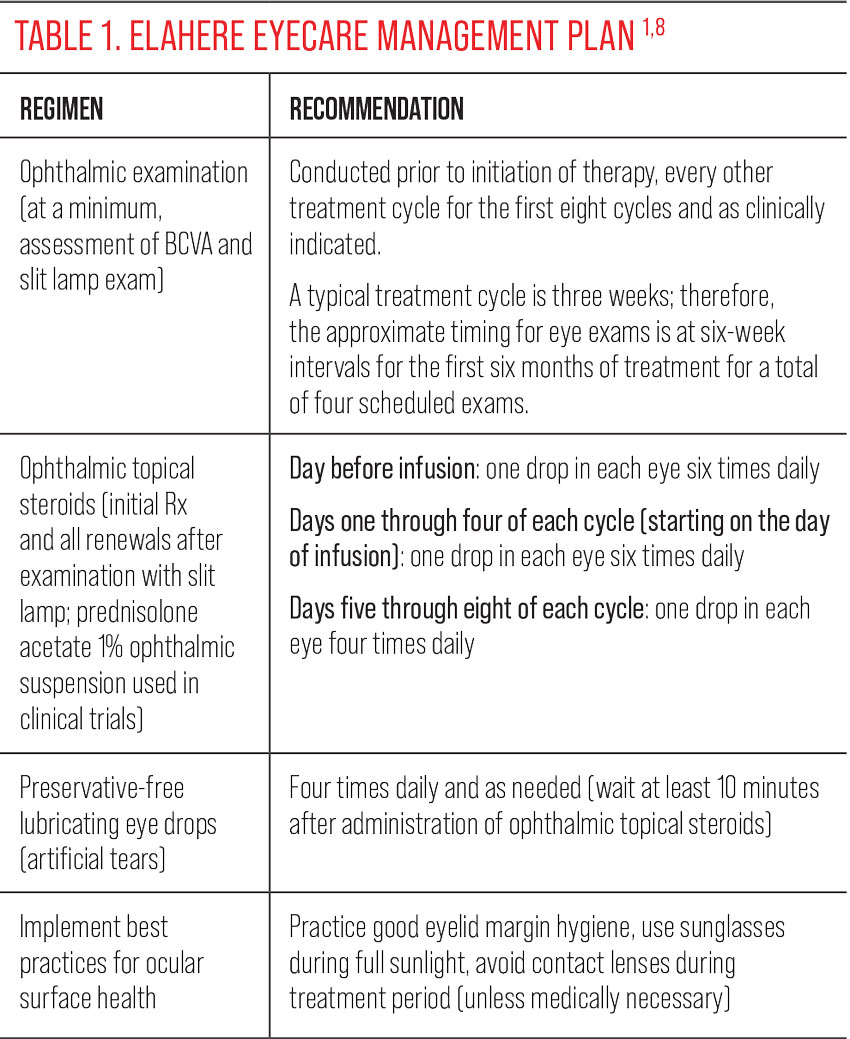
The recommended eyecare management plan for Elahere keratopathy includes three components: prophylactic use of topical steroids, ocular surface disease management, including use of preservative-free artificial tears, and baseline and monitoring eye exams.8
It’s important to note that each ADC carries differing toxicities and therefore eyecare management plans may also differ (Table 1). The pre-Elahere exam is used to establish baseline refractive and ocular health status as a reference point for later evaluation of treatment-related changes, as well as to clear the patient to start topical steroids. This is a great time to offer the patient education on how and when to use their eye drops and to offer pre-treatment of any existing ocular surface disorders. Follow-up exams should include an assessment of BCVA with refraction performed as clinically indicated, along with a slit lamp examination of the anterior segment, paying close attention to the cornea and ocular surface.
Elahere is administered by IV infusion at three-week intervals. The relationship between the extent of exposure to Elahere in the plasma and the development of MECs allows the oncologist to apply dose modifications in response to the development of adverse reactions. The term “dose modification” refers to delaying the next infusion, reducing the dosage level, or, in the case of high-grade events, discontinuation of the treatment. This is primarily determined by the ocular findings. MEC keratopathy would indicate that too much drug is present. The aim is to allow time for the resolution of clinically significant adverse reactions before the next infusion and potentially reduce the severity of subsequent occurrences.1
The dose modification criteria for Elahere includes classification of MECs as confluent or non-confluent. Generally, confluent keratopathy is described as multiple macro-punctate lesions in the corneal epithelium that have coalesced or appear patchy, while non-confluent superficial keratopathy may consist of multiple, distinct micro-punctate lesions in the corneal epithelium that may be numerous or dense, but have not coalesced. Non-confluent keratopathy without a clinically meaningful reduction in vision may require only monitoring and symptom management without requiring delay of the next infusion.
Keratopathy with significant reduction in BCVA (≥ three lines) or confluent keratopathy may warrant dose modification by the oncologist, including holding the next dose until it has resolved to the level of non-confluence. In addition to providing the oncologist with information to guide dose modifications, ODs should manage any concomitant ocular surface disorders.1,8
In the pooled safety analysis, more than half of ocular adverse reactions resolved to grade 1 or better within 14 days without requiring dose modification. Dose modifications were effective in managing these events, with <1% of patients discontinuing Elahere due to ocular adverse events. There were no corneal ulcers, perforations or permanent ocular sequelae reported.1
 |
Building Bridges
Oncologists need our help to assess adverse events that may occur related to the use of ADC’s and to prescribe and monitor prophylactic steroid drops, including evaluating BCVA, IOP and corneal findings observed on slit lamp exam and assessing changes.
Ophthalmic exam findings are used by the oncologist to determine the need for dose modifications in the context of the patient’s overall health status, and ultimately, help to find the best balance between the patient’s vision-related quality of life and their cancer treatment.
Getting involved in the care of women with ovarian cancer is an opportunity to network with oncology teams during a time when the number of novel cancer therapies requiring ophthalmic monitoring is steadily growing, and more importantly, an opportunity to ease a patient’s cancer burden by ensuring that her vision is in good hands.
Thanks to Grace Lytle, OD, for her contributions to this column.
Dr. Karpecki is the director of Cornea and External Disease for Kentucky Eye Institute, associate professor at KYCO and medical director for Dry Eye Institutes of Kentucky and Indiana. He is the Chief Clinical Editor for Review of Optometry and chairman of the affiliated New Technologies & Treatments conferences. A fixture in optometric clinical education, he provides consulting services to a wide array of ophthalmic clients. Dr. Karpecki’s full disclosure list can be found here.
1. Hendershot A, Slabaugh M, Riaz KM, et al. Strategies for prevention and management of ocular events occurring with mirvetuximab soravtansine. Gynecol Oncol Rep. 2023;47:101155. 2. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics. CA Cancer J Clin. 2023;73(1):17-48. 3. Armstrong DK, Alvarez RD, Backes FJ, et al. NCCN Guidelines insights: ovarian cancer, version 3. J Natl Compr Canc Netw. 2022;20(9):972-80. 4. Moore KM, Angelergues A, Konecny GE, et al. Mirvetuximab soravtansine in FRα positive, platinum-resistant ovarian cancer. N Engl J Med. 2023;389(23):2162-74. 5. Kunkler AL, Binkley EM, Mantopoulos D, et al. Known and novel ocular toxicities of biologics, targeted agents and traditional chemotherapeutics. Graefes Arch Clin Exp Ophthalmol. 2019;257(8):1771-81. 6. Corbelli E, Miserocchi E, Marchese A, et al. Ocular toxicity of mirvetuximab. Cornea. 2019;38(2):229-32. 7. Canestraro J, Hultcrantz M, Modi S, et al. Refractive shifts and changes in corneal curvature associated with antibody-drug conjugates. Cornea. 2022;41(6):792-801. 8. Elahere, 2022. Prescribing information. ImmunoGen, Inc. |

