 This past October, I was invited to lecture to the New Zealand Association of Optometrists. I had quite an experience down under, and I am happy to say that optometry is alive and thriving in
This past October, I was invited to lecture to the New Zealand Association of Optometrists. I had quite an experience down under, and I am happy to say that optometry is alive and thriving in
My wife and I toured extensively. We enjoyed the classic grace of

However, there were some things that I didnt see. Nowhere did I see anyone dead or dying in the streets. I did not hear ambulances racing to crowded hospitals, nor did I see any funeral processions heading to overfilled cemeteries. Indeed, I saw none of these, despite the fact that the most commonly prescribed topical ophthalmic antibiotic in
What Is Chloramphenicol?
Chloramphenicol is a bacteriostatic antibiotic that inhibits bacterial protein synthesis.1 However, it can be bactericidal in high concentrations or when used against highly susceptible organisms. Chloramphenicol reversibly binds to the 50S subunit of the 70S ribosome, preventing successful attachment of complete RNA transfer.1
Outside of the
It is available in systemic form as well as a topical ophthalmic 0.5% solution and 1% ointment. Topical dosing is recommended at five times per day. It has a broad spectrum of both gram-positive and gram-negative antibacterial activity, and it is effective against anaerobic organisms, mycoplasma, rickettsia and chlamydia.2-6,7 More than 95% of Haemophilus influenzae, Neisseria meningitides, Neisseria gonorhoeae, Salmonella typhi, Brucella species and Bordetella pertussis are susceptible to chloramphenicol.7 Especially noteworthy are its low rate of clinical and microbiologic resistance and its ability to conquer organisms resistant to more common antibiotics.8-10
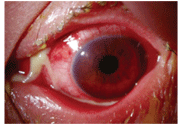
Chloramphenicols efficacy against ocular methicillin-resistant Staphylococcus aureus (MRSA) infections has also been noted.10 It is used extensively throughout the world (with the exception of the United States) to treat acute bacterial infections and corneal trauma.10-15 Also, due to its excellent ocular penetration, chloramphenicol is very popular in surgical prophylaxis.11-14 The topical form is well tolerated, with a low reported incidence of burning and stinging.1
The Fall of Chloramphenicol
Since the inception of systemic chloramphenicol, reports have associated it most notably with aplastic anemia. Fatal aplastic anemia has been associated with systemic chloramphenicol use, but the first case of aplastic anemia associated with topical use was reported in the 1960s.1,7,16 In 1980 and 1982, the first two cases of fatal aplastic anemia linked with topical chloramphenicol use were reported, and another was reported in 1992.17-19
To date, 23 cases of aplastic anemia (the majority were not fatal) have possibly been associated with the use of topical chloramphenicol.7
While topical chloramphenicol use is widespread throughout the world, this possible association with aplastic anemia has curtailed its use in the
What is Aplastic Anemia?
In aplastic anemia, bone marrow does not produce sufficient new blood cells. The marrow suffers from an aplasia that renders it unable to function properly, which results in anemia, with fewer erythrocytes, leukocytes and platelets than normalor fewer than are needed to function properly.
Aplastic anemia is life-threatening; it has a 50% mortality rate.1 The condition can be associated with certain medications or occur idiopathically.20 Idiopathic aplastic anemia has an incidence of one in 524,000, and the presumed systemic chloramphenicol-induced disease occurs in one in 24,500 to 40,800.1
Chloramphenicol-induced anemia ranges from a more common, reversible, dose-dependent toxicitycharacterized by mild anemia, thrombocytopenia and neutropeniato the more severe, irreversible aplastic anemia, involving progressive pancytopenia.1 There may actually be a genetic predisposition to chloramphenicol-induced aplastic anemia, with only a very small segment of the population at risk.1
An Association?
Despite the reported association between chloramphenicol and aplastic anemia, a causal relationship has yet to be firmly established. Many in the medical community are skeptical of claims that chloramphenicol actually causes aplastic anemia.1,7,21-23
Of the 23 possible cases of topical chloramphenicol-induced aplastic anemia, only seven were published; the remainder were never fully investigated.7 In these seven, compelling evidence actually de-emphasizes the role of chloramphenicol. All but one patient had long periods of topical therapy (an average of 13 months), and three patients concurrently used other marrow-toxic medications. Two had concomitant liver disease. Possible genetic predispositions were also found in three patientsfamily history of aplastic anemia, pernicious anemia and leukemia.7
During a 10-year span in the United Kingdom, 11 suspected cases of non-fatal topical chloramphenicol-induced blood dyscrasia were noted in more than 200 million uses.1 This frequency (1:20 million cases) is far less than the 1:100,000 frequency of penicillin-induced anaphylaxis.16 The calculated fatality rate of systemic chloramphenicol is 1:90,000, while that of penicillin is 1:100,000; yet, penicillin is used routinely.1
In another report, the incidence of aplastic anemia among users of ocular chloramphenicol was 0.36 cases per million weeks of treatment. The incidence of idiopathic aplastic anemia among non-users was 0.04 cases per million weeks. Based on this study, an association between ocular chloramphenicol and aplastic anemia could not be excluded; but, the risk was less than one per million treatment courses.22 The minimum total dose of topical chloramphenicol associated with marrow toxicity is 30mg and the minimum duration of exposure is 18 days, but a conventional course of topical chloramphenicol therapy delivers only 10mg to the eye.1
The point here is not to induce resurgence in chloramphenicol use, but to debunk the myths about this drug. I occasionally prescribed chloramphenicol during my training, but I was taught to fear this drug due to the possibility of inducing a fatal reaction. Those who instilled this aversion in me also lamented not being able to use the antibiotic because it was an effective drug.
Topical beta-blockers also have the possibility of fatal reactions in susceptible individuals; yet, these continue to be highly prescribed. Beta-blockers are only avoided in susceptible individuals with bradycardia or pulmonary disease. Similarly, by avoiding chloramphenicol use in patients with family history of aplastic anemia, liver disease or known chloramphenicol sensitivities, this drug could be utilized safely and effectively in many patients. Combine this with its broad spectrum of activity, extremely low resistance rate (Who uses it enough to cause resistance?) and low price, and chloramphenicol could make for an exceptional antimicrobial agent.
Next month, Dr. Kabat returns.
1. McGhee CN, Anastas CN. Widespread ocular use of topical chloramphenicol: Is there justifiable concern regarding idiosyncratic aplastic anaemia? Br J Ophthalmol 1996 Feb;80(2):1824.
2. Usha K, Smitha S, Shah N, et al. Spectrum and the susceptibilities of microbial isolates in cases of congenital nasolacrimal duct obstruction. J AAPOS 2006 Oct;10(5):469-72.
3. Matuska S, Rama P, Cavallero A, et al. Nocardia keratitis: a case report. Eur J Ophthalmol 2006 Jan-Feb;16(1):164-7.
4. Arantes TE, Cavalcanti RF, Diniz Mde F, et al. Conjunctival bacterial flora and antibiotic resistance pattern in patients undergoing cataract surgery. Arq Bras Oftalmol 2006 Jan-Feb;69(1):33-6.
5. Orden Martnez B, Martnez Ruiz R, Milln Prez R. Bacterial conjunctivitis: most prevalent pathogens and their antibiotic sensitivity. An Pediatr (Barc) 2004 Jul;61(1):32-6.
6. Robert PY, Adenis JP. Comparative review of topical ophthalmic antibacterial preparations. Drugs 2001;61(2):175-85.
7. Lam RF, Lai JS, Ng JS, et al. Topical chloramphenicol for eye infections.
8. Egger SF, Ruckhofer J, Alzner E, et al. In vitro susceptibilities to topical antibiotics of bacteria isolated from the surface of clinically symptomatic eyes. Ophthalmic Res 2001 Mar-Apr;33(2):117-20.
9. Altaie R, Fahy GT, Cormican M. Failure of Listeria monocytogenes keratitis to respond to topical ofloxacin. Cornea 2006 Aug;25(7):849-50.
10. Cimolai N. Ocular Methicillin-resistant Staphylococcus Aureus Infections in a Newborn Intensive Care Cohort. Am J Ophthalmol 2006 Jul;142(1):183-4.
11. Gordon-Bennett P, Karas A, Flanagan D, et al. A survey of measures used for the prevention of postoperative endophthalmitis after cataract surgery in the
12. Aslam SA, Sheth HG,
13. Upadhyay MP, Karmacharya PC, Koirala S, et al. The Bhaktapur eye study: ocular trauma and antibiotic prophylaxis for the prevention of corneal ulceration in
14. Rose PW, Harnden A, Brueggemann AB, et al. Chloramphenicol treatment for acute infective conjunctivitis in children in primary care: a randomised double-blind placebo-controlled trial. Lancet 2005 Jul 2-8;366(9479):37-43.
15. Jassim KA, Sequeira RR, Mathur VS. Trends in ophthalmic antimicrobial utilization pattern in
16. Isenberg SJ. The fall and rise of chloramphenicol. J AAPOS 2003 Oct;7(5):307-8.
17. Fraunfelder FT, Bagby GC Jr, Kelly DJ. Fatal aplastic anemia following topical administration of ophthalmic chloramphenicol. Am J Ophthalmol 1982 Mar;93(3):356-60.
18. Abrams SM, Degnan TJ, Vinciguerra V. Marrow aplasia following topical application of chloramphenicol eye ointment. Arch Intern Med 1980 Apr;140(4):576-7.
19. McWhae JA, Chang J, Lipton JH. Drug-induced fatal aplastic anemia following cataract surgery. Can J Ophthalmol 1992 Oct;27(6):313-5.
20. Young NS. Pathophysiologic mechanisms in acquired aplastic anemia. Hematology Am Soc Hematol Educ Program 2006;72-7.
21. Rayner SA, Buckley RJ. Ocular chloramphenicol and aplastic anaemia. Is there a link? Drug Saf 1996 May;14(5):273-6.
22. Laporte JR, Vidal X, Ballarn E, et al. Possible association between ocular chloramphenicol and aplastic anaemiathe absolute risk is very low. Br J Clin Pharmacol 1998 Aug;46(2):181-4.
23. Field D, Martin D, Witchell L. Ophthalmic chloramphenicol: a review of the literature. Accid Emerg Nurs 1999 Jan;7(1):13-7.

