The prevalence of dry eye disease (DED) is rising around the world and with that comes a plethora of new treatments. Given its multifactorial causes, chronic nature and potential for progression, ongoing persistence with treatment is required. In treating DED, optometrists must understand the pharmacology of the disease and the importance of reducing inflammation. It’s is the chief culprit in dry eye, and in order to fight it, we have to understand how anti-inflammatories work.
The Tear Film and Ocular Surface Society’s (TFOS) Dry Eye Workshop II (DEWS II) definition recognizes the impact of inflammation to the ocular surface, saying, “Dry eye is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.”1
Several therapeutic agents are available to break the vicious circle of dry eye and help prevent chronic disease progression, and there is also a wide variety of non-pharmacological interventions with the potential to reduce inflammation.
We will review the pathogenesis of inflammation in DED, what perpetuates it and how to mitigate it, along with the therapies currently available. With so many tools in our toolbox, there’s never been an easier way to knock out inflammation.
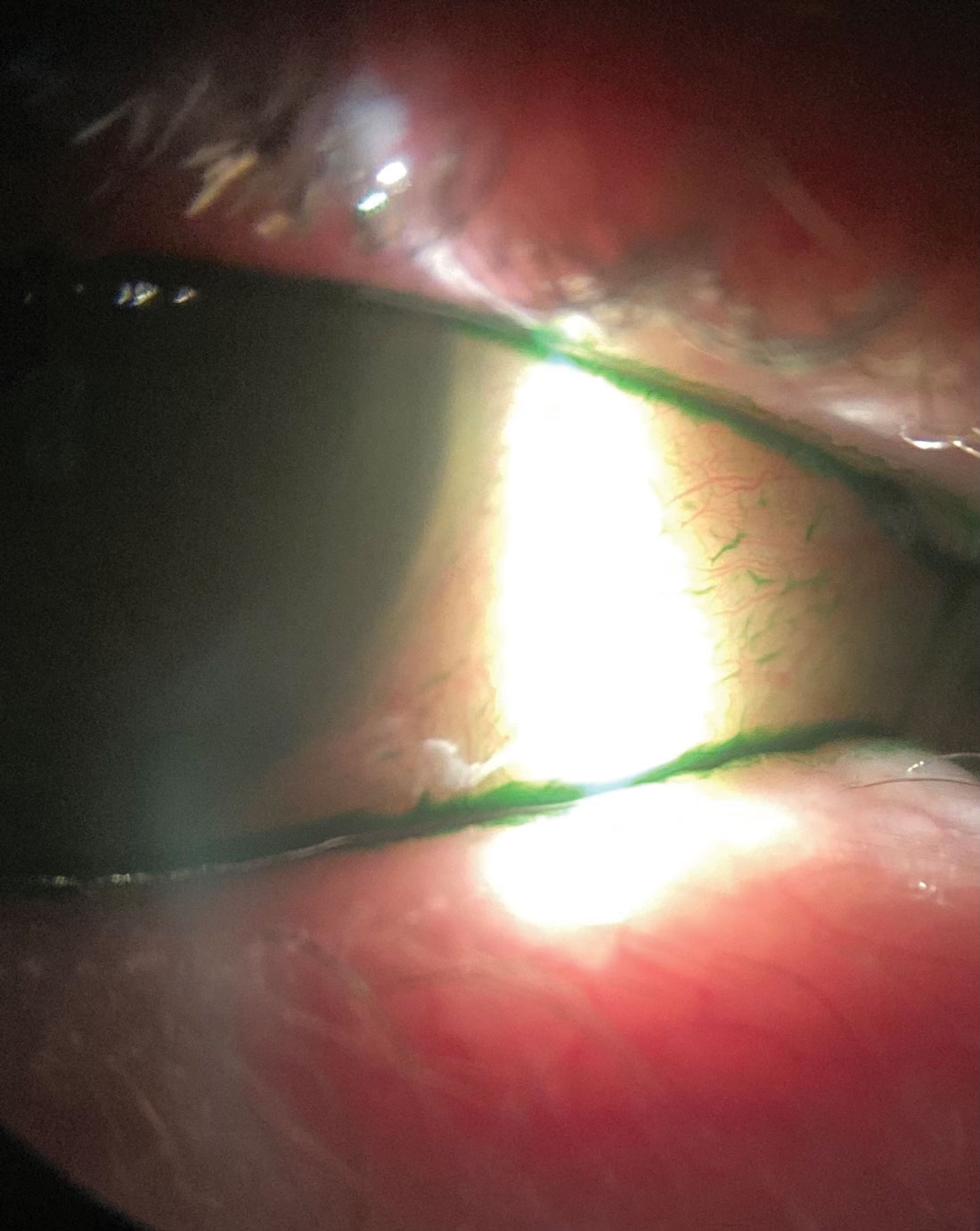 |
Lissamine green vital dye, shown here in a dry eye patient with inflammation, helps visualize devitalized cells on the conjunctiva. Click image to enlarge. |
Inflammation's Role in DED
The central mechanism of DED is a vicious cycle where inflammation plays a key role. The inflammation may be stimulated either by desiccating or hyperosmolar stress—both of which lead to ocular surface damage.2
A growing body of research on the role of inflammation in the pathogenesis of DED has led to the recognition of dysregulation of immune responses on the ocular surface.3 Regardless of the etiology of DED, both chronic inflammation (T-cell mediated immune response) and tear hyperosmolarity are the most common factors in its chronicity.2
Hyperosmolarity can be caused by many factors, including destruction or degeneration of the lacrimal gland, conjunctiva and meibomian glands, damage to corneal nerves (in the case of ophthalmic surgery, corneal infections, long-term contact lens abuse or long-term topical medication use), reduced tear production due to systemic medications (such as beta blockers, oral antihistamines, birth control pills and hormone replacement therapy) and meibomian gland disease.2
Hyperosmolarity induces inflammation in human limbal epithelial cells by increasing expression and production of pro-inflammatory cytokines and chemokines such as IL-1b, TNF-α and IL-8.4 Interleukin (IL)-1 is one of the most widely studied cytokines accompanying dry eye. An increase in the pro-inflammatory forms of IL-1 (IL-1α and mature IL-1β) and a decrease in the biologically inactive precursor IL-1β have been found in the tear film of dry eye patients.5
Infiltration of T-cells into the lacrimal functional unit (which includes the lacrimal gland, goblet cells in the conjunctiva and meibomian glands) is known to result in chronic inflammation.6 Inflammatory mediators released from recruited T-cells and tear hyperosmolarity accentuate cellular damage and loss of epithelial and goblet cells, leading to a vicious cycle of tear film instability and chronic inflammation.7 Throughout the inflammatory response, immune cells release proinflammatory cytokines and chemokines, which recruit more immune cells and eventually results in a vicious cycle of inflammation that does not resolve.2
An appreciation of the basic immunological factors associated with DED is essential for appropriate management of patients with the disease.8 DED immunopathophysiology is characterized by four stages: initiation, amplification, recruitment and re-initiation.9 Dessicating stress leading to hyperosmolarity is a great example of the initiation of the vicious circle of DED.2
Dry eye patients (whether with a concomitant autoimmune disease or not) have conjunctival inflammation manifested by T-cell infiltrates and upregulation of CD3, CD4 and CD8. They also demonstrate lymphocyte activation markers CD11a and HLA-DR.10 Inflammation in dry eye disease is mediated by lymphocytes.11 MAP kinases were found to stimulate the production of inflammatory cytokines, including IL-1, TNF-α and MMP-9, and thereby causing ocular surface damage.12
Vital dyes are also valuable in the detection of ocular surface damage often seen alongside inflammation. The use of sodium fluorescein or lissamine green vital dyes is particularly helpful in identifying and visualizing the devitalized cells on the conjunctiva.13
 |
This patient demonstrates moderate staining of corneal epithelium with sodium fluorescein. Click image to enlarge. |
How to Mitigate Inflammation
It is widely recognized that inflammation has a significant role in dry eye disease. It promotes the breakdown of the ocular surface and leads to symptoms of irritation and, commonly, visual disturbance. Anti-inflammatory treatments are a mainstay of the management of dry eye disease, as they inhibit the expression of inflammatory mediators on the ocular surface and act to restore a healthy tear film and decrease the signs and symptoms.14
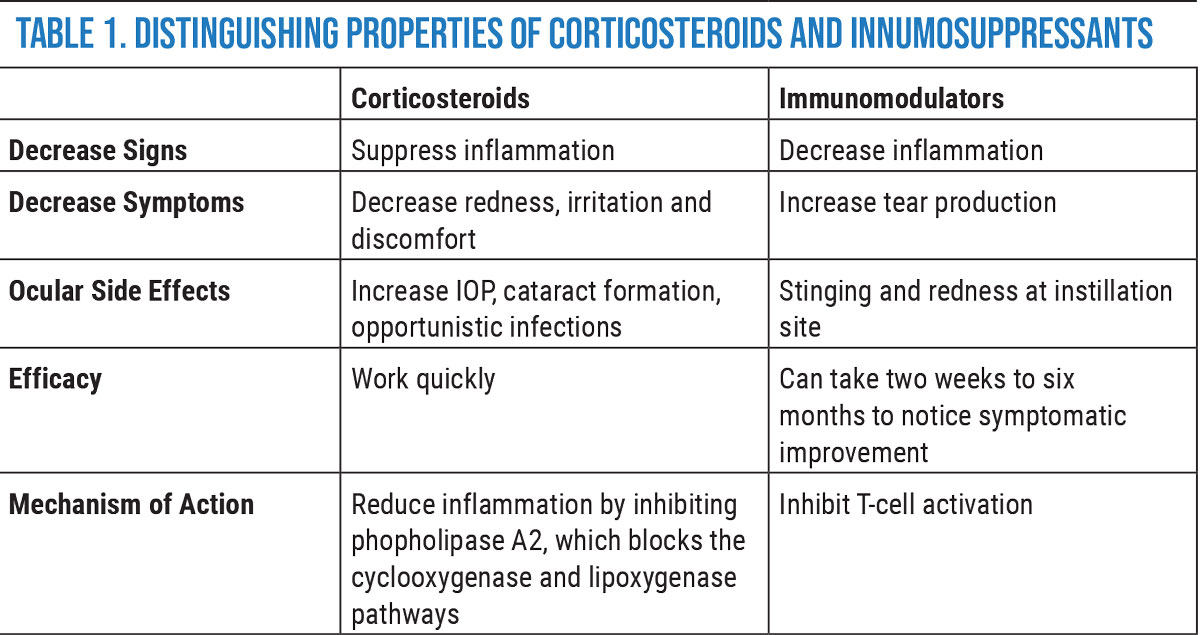
Current anti-inflammatory therapies include corticosteroids (lotepredenol, prednisolone, fluoromethalone and dexamethasone), immunomodulators (cyclosporine and liftegrast) and oral tetracyclines (doxycyline). Let’s briefly review how these modalities interrupt the inflammatory process.
Corticosteriods. These agents work by suppressing cellular infiltration, capillary dilation, fibroblast proliferation and collagen deposition, and they also stabilize intracellular and extracellular membranes.14 Corticosteroids also interfere with the synthesis of pro-inflammatory molecules.15 Several clinical studies have demonstrated the effectiveness of topical steroids for treatment of dry eye.16
While topical corticosteroids demonstrate effective interruption of the inflammatory and immune response cycle of DED, long-term use can present complications such as ocular hypertension, cataract formation and opportunistic infections.
Eysuvis. Although corticosteroids are an effective treatment for DED, long-term use is associated with cataract development, increased intraocular pressure (IOP) and opportunistic infections.17 Eysuvis (loteprednol etabonate 0.25%,) an ocular corticosteroid, breaks down rapidly after administration to the ocular surface tissues and reduces the risks associated with other topical steroids, as well as potentially decreases harmful side effects such as elevated IOP and cataract formation.18
Loteprednol was formulated based on the “inactive metabolite approach,” where it is metabolized by hydrolysis. In both in vitro and in vivo metabolism of loteprednol, an inactive, hydrophilic metabolite was easily eliminated from the body.19 Loteprednol etabonate was retro-metabolically engineered 30 years ago to reduce the common risks of topical ocular steroids, including IOP elevation and cataract formation.20 The Lotemax family of loteprednol-based products has been long used off-label in dry eye patients.
The tear film’s ability to efficiently remove foreign particles during blinking can pose challenges for topical drug delivery. Traditional eye medications in the form of drop solutions and suspensions are cleared from the ocular surface by blinking before the drug is able to penetrate conjunctival and corneal epithelium.18 On the ocular surface, the function of the mucus barrier is to protect cellular surfaces and maintain water balance.21
Eysuvis is an ophthalmic nanosuspension that delivers loteprednol to the anterior eye using mucus-penetrating particles (MPPs). This method has been shown to efficiently penetrate the mucus barrier and reach the ocular surface tissues quickly.18 The MPP formulation of loteprednol etabonate ophthalmic suspension has favorable properties for treatment of ocular surface disease.18 In pre-clinical studies, 0.5% loteprednol vs. 0.4% loteprednol in MPP formulation was shown to have a 3.6-fold higher concentration in the corneal epithelium after just five minutes.22
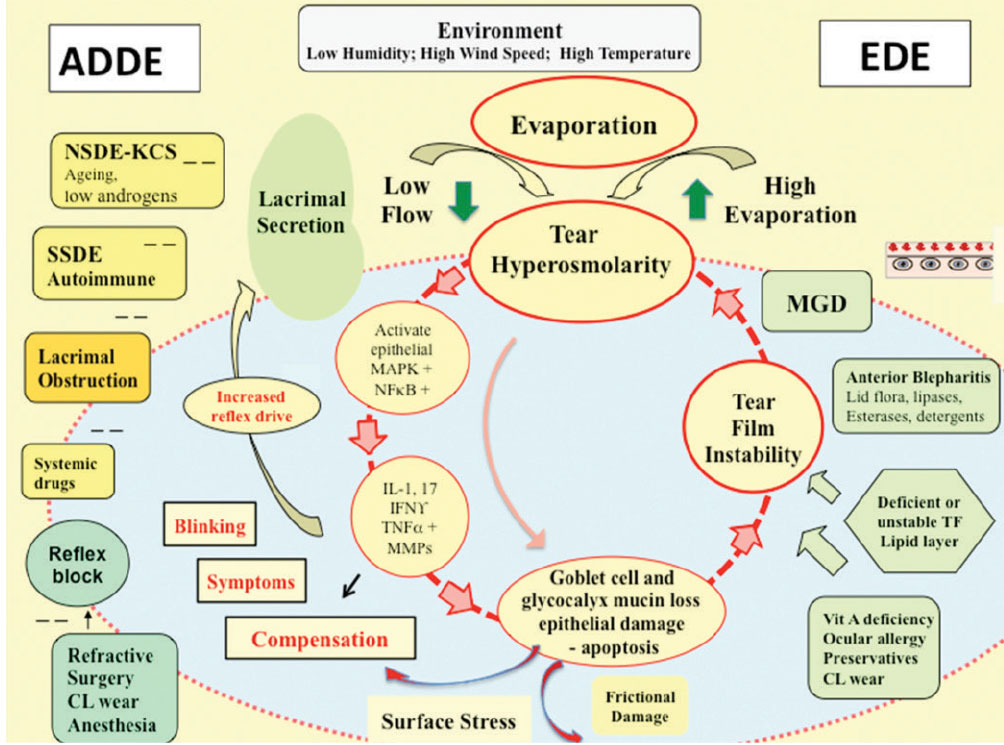 |
The “vicious cycle” of inflammation that drives chronic dry eye. Click image to enlarge. |
Dry Eye Flares. The TFOS DEWS II report describes an initial presentation of DED that may involve intermittent symptoms or “emerging episodic dry eye.”23 However, there’s evidence in a variety of settings for the existence of periodic flares of DED in the context of ongoing disease.18
Patients who present with mild signs and symptoms often do not require chronic immunomodulatory therapy, such as cyclosporine A or lifitegrast, as some patients may also experience periodic flares on a seasonal or episodic basis and would benefit from short-term therapy when their symptoms flare-up.16 DED flares may occur episodically in response to specific triggers, such as low humidity, air conditioning and windy conditions.24
In patients with episodic disease, appropriate therapy at the time of a flare-up could potentially break the vicious circle of inflammation early in its process and help prevent further damage to the ocular surface. Flares of otherwise mild DED may benefit from short-term steroid therapy.18
When an episode of ocular surface discomfort and a flare of DED is diagnosed, a short course of Eysuvis may relieve the patient’s symptoms and potentially calm the vicious cycle of inflammation.18 The Stride 1 Phase III clinical trial with 914 patients demonstrated a clinically significant change in ocular discomfort score after only eight days.25
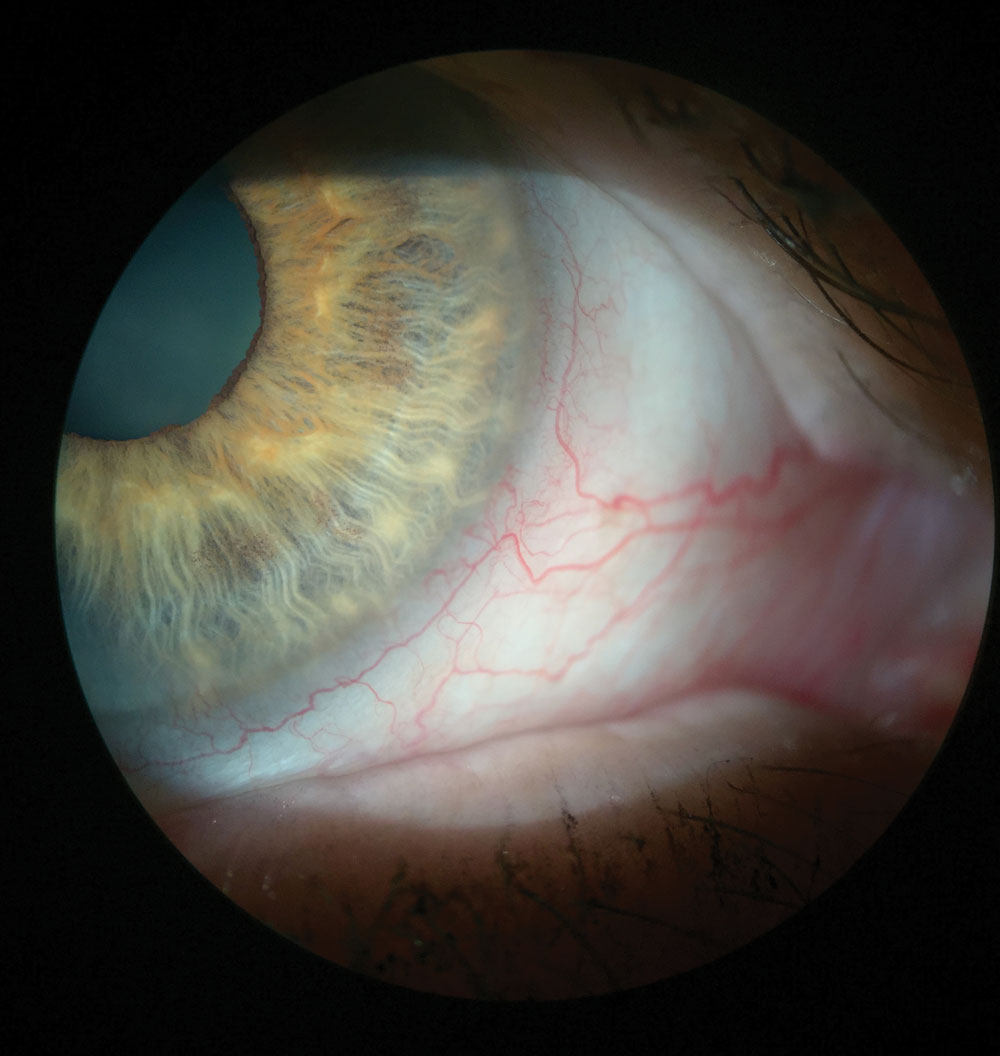 |
This patient’s injected blood vessels indicate inflammation, which can be seen without any vital dyes. Click image to enlarge. |
Cyclosporine A. Topical formulations of cyclosporine A (CsA) provide a broad-based approach to DED treatment by decreasing inflammation and improving the integrity of the ocular surface with few systemic effects.9 Due to CsA 0.05% immunomodulating effects, it can be the therapy of choice for patients with DED and an underlying autoimmune disease vs. using a corticosteroid.13 The immunomodulator is the better treatment due to the chronic nature of autoimmune disease because it can control the inflammation over long periods of time.13
CsA has principal pharmacologic action of suppressing the activation and function of T-lymphocytes, acting as an immunosuppressant and inhibitor of cell death.14 The reduction in inflammation, via inhibition of T-cell activation and down-regulation of inflammatory cytokines in the conjunctiva and lacrimal gland, is thus thought to allow enhanced tear production.26 Topical cyclosporine also increases goblet cell density and decreases epithelial cell apoptosis.9,27
Cyclosporine A is a calcineurin inhibitor that exerts immunomodulatory effects by blocking T-cell infiltration, activation and the subsequent release of inflammatory cytokines.28 The action of CsA on T-cells is the primary mechanism for DED symptom improvement; however, its effects may extend beyond T-cell modulation. Twice-daily treatment for two weeks with CsA 0.05% decreased expression of proinflammatory cytokines and chemokines IL-1β, TNF-α, IL-6, intercellular adhesion molecule 1 and vascular cell adhesion molecule 1.29
CsA reduces the underlying inflammation associated with DED that interferes with tear production and has fewer ocular complications than steroids.
Restasis. Topical CsA was developed to increase tear production in patients with DED who did not respond sufficiently to conservative treatments such as ocular lubricants and lid hygiene. Benefits of using CsA 0.05% has been shown to improve tear production, tear break-up time, and corneal and conjunctival staining scores.31 Based on BID dosing of CsA 0.05% for three months, 72% of participants were satisfied with their results and 57.2% reported a reduction to mild to no symptoms.32
Restasis (cyclosporine A 0.05%) increases tear production in patients whose tear production is presumed to be suppressed due to ocular inflammation associated with DED.16 Restasis demonstrated improvements observed in DED increased Schirmer’s score and decreased conjunctival staining scores.31 Additionally, using CsA to decrease ocular inflammation helped reduce mechanical stressors on the eye and improved epithelial integrity.33 Improvement in the reduction of persistent epithelial defects and reduction in episodes of recurrent corneal erosions occurred after two months of twice-daily dosing of CsA 0.05%, even though these patients had previously been resistant to therapy with corticosteroids.33
Restasis is safe to use long-term, but can take up to three months before patients notice any improvement.34 Clinical results show that the time to onset of reduction in ocular surface staining and improvement in visual performance with Restasis is on average six months.35 Such long lead times to initial efficacy may interfere with patient compliance with treatment in DED, but continued usage is important to attain therapeutic levels of CsA in ocular tissues.36
Self-reported adherence with the prescribed twice-daily regimen of topical CsA 0.05% was associated with more rapid onset of reported increase in tear production, as well as greater patient satisfaction and willingness to continue therapy.37 However, studies have shown that patients with severe dry eye may require more frequent dosing of Restasis than twice daily.38 Mean corneal fluorescein staining scores and no new-onset symptoms of burning and irritation were reported in participants with graft-vs-host disease and primary or secondary Sjögren’s syndrome who increased their dosage of CsA 0.05% from BID to three to four times daily.38 Thus, newer cyclosporine drugs have come to market to increase the concentration of CsA on the cornea and conjunctiva.14
 |
| Click image to enlarge. |
Cequa. A recently introduced cyclosporine product, Cequa (CsA 0.09%) is an aqueous nanomicellar formulation that increases tear production in patients with DED. It aims to increase CsA bioavailability and reduce adverse reactions.39
Cequa is different from Restasis due to its nanomicellar delivery system. Hydrophobic interactions of core-forming units drive the micelle formation with a water-insoluble or hydrophobic core and an outer water-soluble or hydrophilic shell. Thus, these nanomicelles are more bioavailable in the precorneal tear film.36 Aqueous CsA nanomicelle carriers produce rapid improvement in objective signs of DED such as corneal and conjunctival staining as early as four weeks.30
The aqueous nanomicellar approach used in Cequa may help enhance and maximize tissue availability of CsA. Therefore, the patient will have improved comfort and compliance and potentially better clinical outcomes in treatment.30 This nanomicellar formulation allows the medication to penetrate the ocular surface faster and get to work sooner, while reducing the adverse reactions of stinging and burning felt by patients taking Restasis.
Lifitegrast. This agent (brand name Xiidra) targets inflammation by inhibiting T-cell recruitment, T-cell activation and subsequent cytokine release.40
Lifitegrast 5% is a small-molecule integrin antagonist that inhibits T-cell mediated inflammation by blocking the binding of two important cell surface proteins (lymphocyte function-associated antigen 1 and intercellular adhesion molecule 1), thus lessening overall inflammatory responses.40 The role of T-cells is pivotal in the development of cell-mediated immune responses in the eye. Specifically, CD4 positive (+) T helper (TH) 1 and TH17 T cells have been identified as mediators of ocular surface inflammation in DED.10
Recruitment and activation of these T-cells at the ocular surface leads to the release of pro-inflammatory cytokines. It is these cytokines that contribute to the damage seen in the ocular surface of DED patients. Therapies targeting T-cells will provide a more efficient means to treat DED.
Since lifitegrast works to inhibit T-cell recruitment and activation, patients taking Xiidra felt relief in as little as two weeks. The majority saw improvement in six weeks and it took others as long as 12 weeks, whereas patients taking Restasis would wait up to three months to feel a similar relief in the reduction of corneal and conjunctival staining.41,42
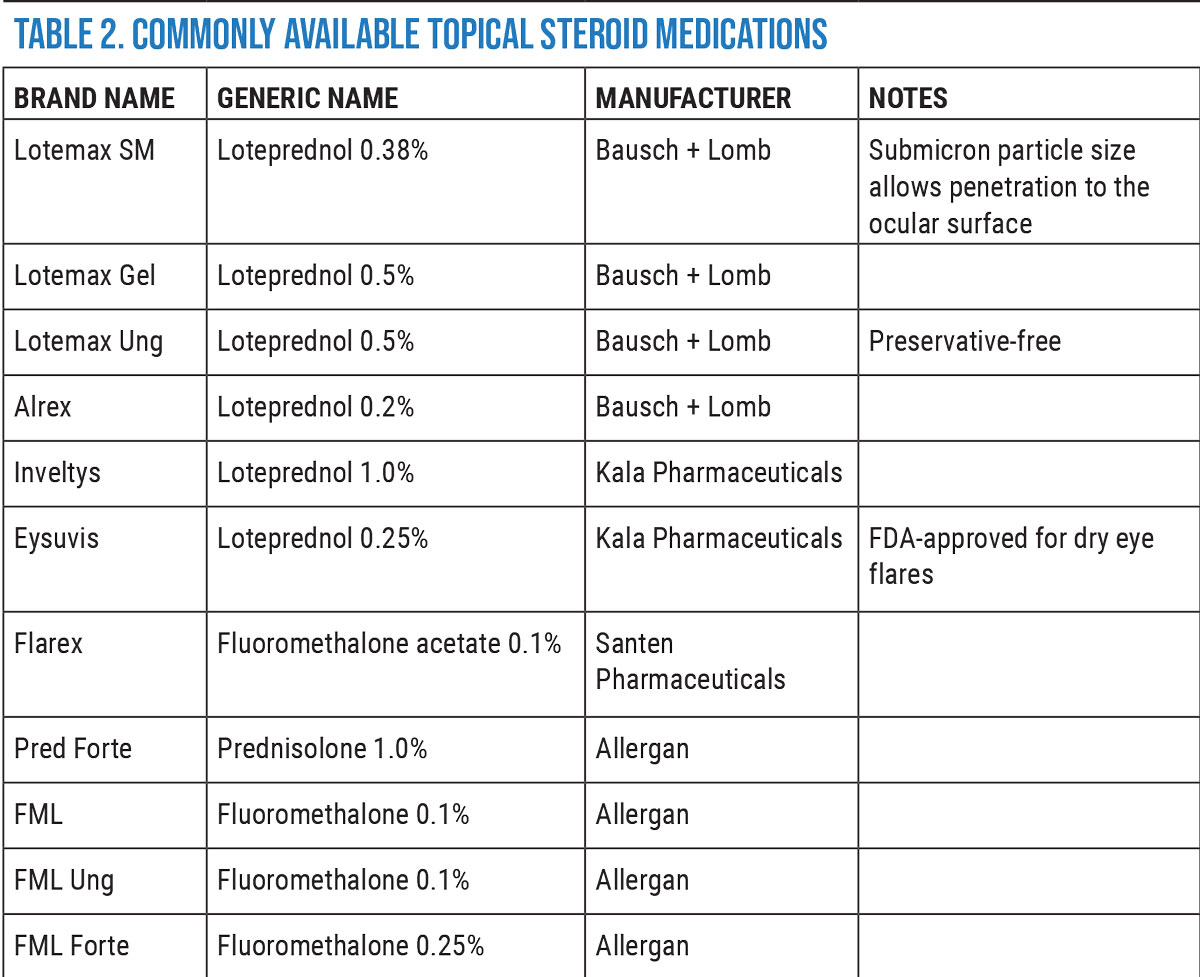 |
| Click image to enlarge. |
Doxycycline. Tetracycline derivatives uniquely possess both antibacterial and anti-inflammatory properties.43 Tetracyclines, such as doxycycline hyclate, present immunomodulating properties that inhibit leukocyte movement during inflammation by preventing calcium-dependent microtubular assembly and lymphocytic proliferation.44 Doxycycline reduces inflammation by decreasing the production of pro-inflammatory cytokines.
In particular, doxycycline has been shown to inhibit c-Jun N-terminal kinase and extracellular signal-related kinase mitogen-activated protein kinase signaling in epithelial cells of the ocular surface exposed to hyperosmolar stress, downregulating the expression of CXCL8 and pro-inflammatory cytokines IL-1β and TNF.43 Doxycycline hyclate works well in the eye due to its high lipophilicity, which allows it to cross multiple membranes to reach target molecules.45
Oral doxycycline can be used long term in low doses to control inflammation on the eye. Studies have shown doses of 40mg daily (or 20mg BID) are effective for anti-inflammatory effects.44 In a study of eight weeks of oral doxycycline hyclate 20mg dosed BID, there was 80% to 100% clearance of inflammatory lesions and 50% reduction in erythema in patients with rosacea.44 The antimicrobial mechanism will not be in action at this sub-antimicrobial dose; therefore, it spares healthy bacteria and maintains the body’s microbiome.45
Doxycycline has been reported to be effective in patients with ocular rosacea by reducing irritation symptoms, improving tear film stability and decreasing the severity of ocular surface disease.46
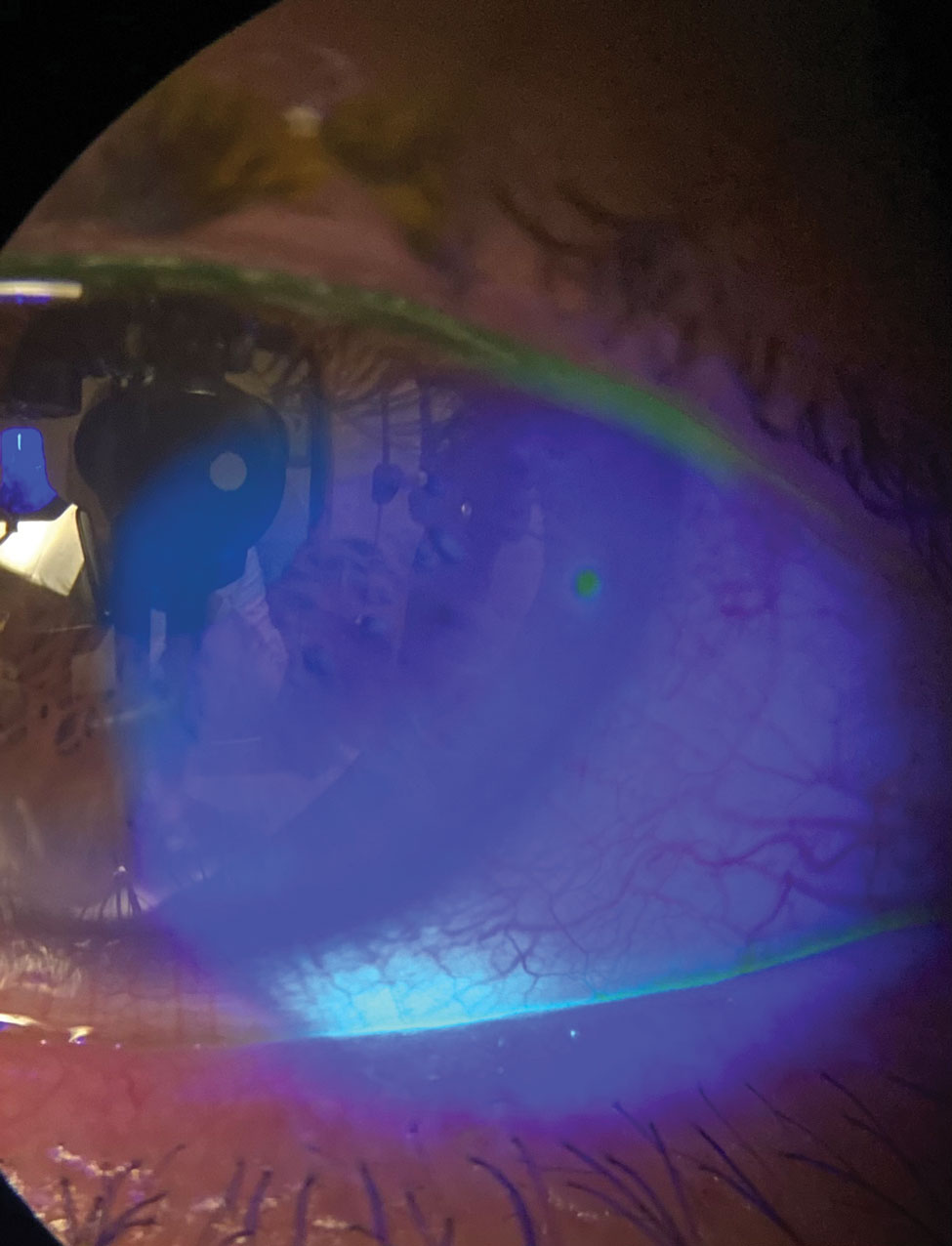 |
Seen here is a 2+ conjunctival injection. This redness is a sign of inflammation. Click image to enlarge. |
Combining Therapies
It is helpful to combine anti-inflammatory treatments to help patients feel comfortable more quickly. Loteprednol works quickly and targets T-cells. Cyclosporine prevents T-cell recruitment but may take several weeks to begin working.9 These two mechanisms of action complement one another and, when used together, provide fast relief and long-term safety.47
Corticosteroids are often used in conjunction with immunomodulators if there’s significant inflammation present on the ocular surface.13
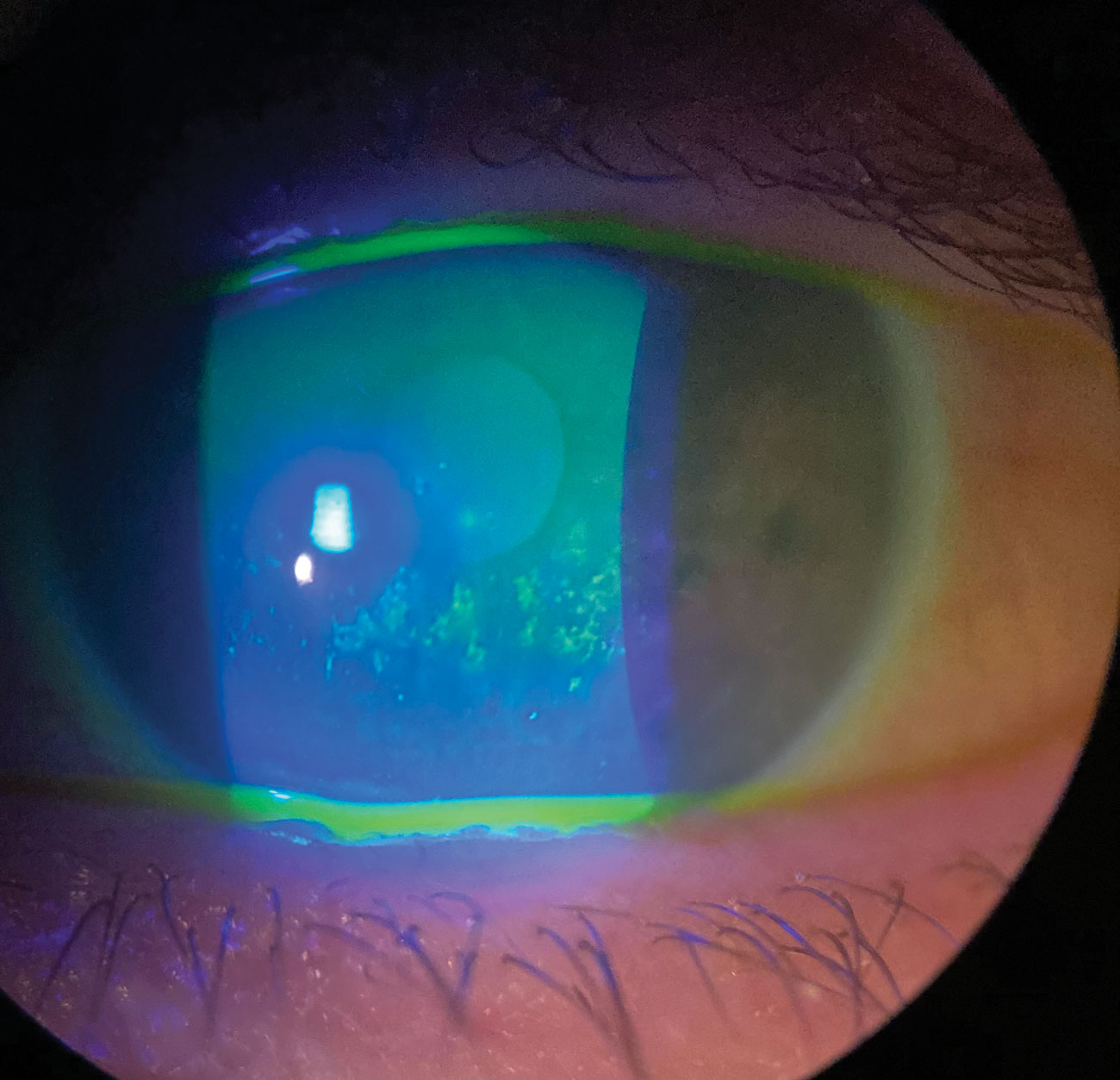 |
A 2+ band of corneal punctate epithelial erosion in a patient with severe keratoconjunctivitis sicca. Click image to enlarge. |
Here are three ways topical steroids can be effective in treating DED:
(1) Alongside an immunomodulator: Eysuvis or Lotemax may be a helpful adjunctive therapy for patients receiving chronic immunomodulatory treatment for DED, especially those with underlying autoimmune or inflammatory conditions.18
(2) At initiation of immunomodulatory therapy: When a dry eye patient starts topical immunomodulatory therapy, the loteprednol-based corticosteroid products have the potential to be used as induction therapy to quell ocular surface inflammation and DED symptoms until the new therapeutic agent takes effect.18
(3) Breakthrough symptoms: Patients using chronic immunomodulatory therapy for DED may still experience periodic episodes of breakthrough symptoms. Topical steroid use could be effective in pulsed doses to treat episodic DED signs and symptoms.18
In addition, there is evidence that doxycycline can be used alongside either corticosteroids or immunomodulators without reducing the effectiveness of either treatment.48 Doxycycline can be used long-term due to its good safety profile and low incidence of bacterial resistance to it at sub-acute dosages.49 It also pairs nicely with other topical anti-inflammatory properties and does not interfere with the absorption of other medications on the ocular surface.50
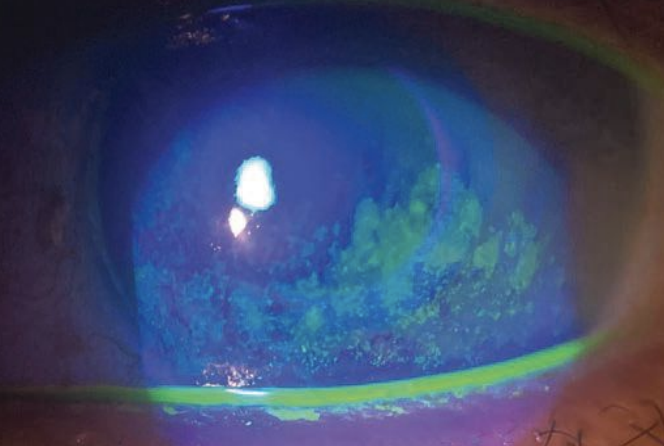 |
|
Here is another patient with severe keratoconjunctivitis sicca, this one with active fluorescein staining. Click image to enlarge. |
Takeaways
Reducing or eliminating inflammation will not just help reduce pain and irritation in the eyes, but will give your patients some much needed relief. To get there, it’s important to manage DED appropriately and facilitate realistic patient expectations—both of which are necessary to ensure patient satisfaction and compliance with their treatment.
Educating your patients is vital to DED management and should convey the complex and chronic nature of the disease, as well as the potential for progression. Though results will likely not be seen immediately, it’s imperative that the duration of medication be fulfilled to ensure inflammation is kicked to the curb.
Dr. Theriot practices at a multi-specialty eye clinic in Shreveport, LA. Her clinical practice covers a broad spectrum of ocular care with a unique clinical focus on ocular surface disease and dry eye. She received her doctorate in optometry from the University of California at Berkeley, School of Optometry, and completed her residency at the State University of New York, College of Optometry. She is a consultant for Novartis and key opinion leader for Sun Pharmaceuticals and Kala Pharmaceuticals.
1. TFOS International Dry Eye WorkShop (DEWS II). Ocular Surf. 2017;15(6):269-650. 2. Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438-510. 3. Wei Y, Asbell PA. The core mechanism of dry eye disease is inflammation. Eye Contact Lens. 2014;40(4):248-56. 4. Li DQ, Luo L, Chen Z, et al. JNK and ERK MAP kinases mediate induction of IL-1beta, TNF-alpha and IL-8 following hyperosmolar stress in human limbal epithelial cells. Exp Eye Res. 2006;82(4):588-96. 5. Solomon A, Dursun D, Liu Z, et al. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42(10):2283-92. 6. Periman LM, Perez VL, Saban DR, et al. The immunological basis of dry eye disease and current topical treatment options. J Ocul Pharmacol Ther. 2020;36(3):137-46. 7. Zhang X, M VJ, He X, et al. Dry eye management: targeting the ocular surface microenvironment. Int J Mol Sci. 2017;18(7):1398. 8. Yagci A, Gurdal C. The role and treatment of inflammation in dry eye disease. Int Ophthalmol. 2014;34(6):1291-301. 9. Periman LM, Mah FS, Karpecki PM. A review of the mechanism of action of cyclosporine A: the role of cyclosporine A in dry eye disease and recent formulation developments. Clin Ophthalmol. 2020;14:4187-200. 10. Stern ME, Gao J, Schwalb TA, et al. Conjunctival T-cell subpopulations in Sjögren’s and non-Sjögren’s patients with dry eye. Invest Ophthalmol Vis Sci. 2002;43(8):2609-14. 11. Kunert KS, Tisdale AS, Stern ME, et al. Analysis of topical cyclosporine treatment of patients with dry eye syndrome: effect on conjunctival lymphocytes. Arch Ophthalmol. 2000;118(11):1489-96. 12. Luo L, Li DQ, Doshi A, et al. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45(12):4293-301. 13. Hessen M, Akpek EK. Dry eye: an inflammatory ocular disease. J Ophthalmic Vis Res. 2014;9(2):240-50. 14. Dang V. Sizing up anti-inflammatories in dry eye disease: a practical guide for optometrists applying these medications. Rev Optom. April 15, 2018;155(4):62. 15. Rhen T, Cidlowski JA. Anti-inflammatory action of glucocorticoids - new mechanisms for old drugs. N Engl J Med. 2005;353(16):1711-658. 16. Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575-628. 17. Ambroziak AM, Szaflik J, Szaflik JP, et al. Immunomodulation on the ocular surface: a review. Cent Eur J Immunol. 2016;41(2):195-208. 18. Gupta PK, Venkateswaran N. The role of KPI-121 0.25% in the treatment of dry eye disease: penetrating the mucus barrier to treat periodic flares. Ther Adv Ophthalmol. 2021;13:25158414211012797. 19. Sigurdsson HH, Kirch J, Lehr CM. Mucus as a barrier to lipophilic drugs. Int J Pharm. 2013;453(1):56-64. 20. Alberth M, Wu WM, Winwood D, et al. Lipophilicity, solubility and permeability of loteprednol etabonate: a novel, soft anti-inflammatory corticosteroid. J Biopharma Sci. 1991;2:115-25. 21. Sigurdsson HH, Kirch J, Lehr CM. Mucus as a barrier to lipophilic drugs. Int J Pharm. 2013;453(1):56-64. 22. Schopf L, Enlow E, Popov A, et al. Ocular pharmacokinetics of a novel loteprednol etabonate 0.4% ophthalmic formulation. Ophthalmol Ther. 2014;3(1-2):63-72. 23. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf 2017;15:(3):276-83. 24. Iyer JV, Lee SY, Tong L. The dry eye disease activity log study. ScientificWorldJournal. 2012;2012:589875. 25. Holland E, Nichols K, Foulks G, et al. Safety and efficacy of KPI-121 ophthalmic suspension 0.25% for dry eye disease in four randomized controlled trials. Presented at: AAO 2020: November 13-15, 2020; virtual meeting. 26. Stevenson D, Tauber J, Reis BL. Efficacy and safety of cyclosporine A ophthalmic emulsion in the treatment of moderate to severe dry eye disease: a dose-ranging, randomized trial. The Cyclospoine A Phase 2 Study Group. Ophthalmology. 2000;107(5):967-74. 27. Kunert KS, Tisdale AS, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002;120(3):330-7. 28. Gao J, Sana R, Calder V, et al. Mitochondrial permeability transition pore in inflammatory apoptosis of human conjunctival epithelial cells and T cells: effect of cyclosporin A. Invest Ophthalmol Vis Sci. 2013;54(7):4717-33. 29. Bang SP, Yeon CY, Adhikari N, et al. Cyclosporine A eyedrops with self-nanoemulsifying drug delivery systems have improved physicochemical properties and efficacy against dry eye disease in a murine dry eye model. PLoS One. 2019;14(11):e0224805. 30. Jerkins GW, Pattar GR, Kannarr SR. A review of topical cyclosporine A formulations - a disease-modifying agent for keratoconjunctivitis sicca. Clin Ophthalmol. 2020;14:481-9. 31. Schultz C. Safety and efficacy of cyclosporine in the treatment of chronic dry eye. Ophthalmol Eye Dis. 2014;6:37-42. 32. Byun YS, Rho CR, Cho K, et al. Cyclosporine 0.05% ophthalmic emulsion for dry eye in Korea: a prospective, multicenter, open-label, surveillance study. Korean J Ophthalmol. 2011;25(6):369-74. 33. Napoli PE, Braghiroli M, Iovino C, et al. A study of refractory cases of persistent epithelial defects associated with dry eye syndrome and recurrent corneal erosions successfully treated with cyclosporine A 0.05% eye drops. Drug Des Devel Ther. 2019;13:2001-8. 34. Kymionis GD, Bouzoukis DI, Diakonis VF, Siganos C. Treatment of chronic dry eye: focus on cyclosporine. Clin Ophthalmol. 2008;2(4):829-36. 35. Stonecipher KG, Torkildsen GL, Ousler GW III, et al. The IMPACT study: a prospective evaluation of the effects of cyclosporine ophthalmic emulsion 0.05% on ocular surface staining and visual performance in patients with dry eye. Clin Ophthalmol. 2016;10:887-95. 36. Vaishya RD, Khurana V, Patel S, Mitra AK. Controlled ocular drug delivery with nanomicelles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014;6(5):422-37. 37. Trattler W, Katsev D, Kerney D. Self-reported compliance with topical cyclosporine emulsion 0.05% and onset of the effects of increased tear production as assessed through patient surveys. Clin Ther. 2006;28:1848-56. 38. Dastjerdi MH, Hamrah P, Dana R. High-frequency topical cyclosporine 0.05% in the treatment of severe dry eye refractory to twice-daily regimen. Cornea. 2009;28(10):1091-6. 39. Ames P, Galor A. Cyclosporine ophthalmic emulsions for the treatment of dry eye: a review of the clinical evidence. Clin Investig (Lond). 2015;5(3):267-85. 40. Perez VL, Pflugfelder SC, Zhang S, et al. Lifitegrast, a novel integrin antagonist for treatment of dry eye disease. Ocul Surf. 2016;14(2):207-15. 41. Abidi A, Shukla P, Ahmad A. Lifitegrast: A novel drug for treatment of dry eye disease. J Pharmacol Pharmacother. 2016;7(4):194-8. 42. Wirta DL, Torkildsen GL, Moreira HR, et al. A clinical phase II study to assess efficacy, safety and tolerability of waterfree cyclosporine formulation for treatment of dry eye disease. Ophthalmology. 2019;126(6):792-800. 43. Solomon A, Rosenblatt M, Li D, et al. Doxycycline inhibition of interleukin-1 in the cornea epithelium. Invest Ophthalmol Vis Sci. 2000;41(9):2544-57. 44. Valentín S, Morales A, Sánchez JL, Rivera A. Safety and efficacy of doxycycline in the treatment of rosacea. Clin Cosmet Investig Dermatol. 2009;12:129-40. 45. Patel RS, Parmar M. Doxycycline Hyclate. www.ncbi.nlm.nih.gov/books/NBK555888 StatPearls Publishing. January 6, 2022. Accessed February 20, 2022. 46. Arman A, Demirseren DD, Takmaz T. Treatment of ocular rosacea: comparative study of topical cyclosporine and oral doxycycline. Int J Ophthalmol. 2015;8(3):544-9. 47. Karpecki P. Optimizing the management of dry eye inflammation. www.optometricmanagement.com/issues/2007/july-2007/optimizing-the-management-of-dry-eye-inflammation. Optometric Managment. July 1, 2007. Accessed January 23, 2022. 48. Wang L, Tsang H, Coroneo M. Treatment of recurrent corneal erosion syndrome using the combination of oral doxycycline and topical corticosteroid. Clin Exp Ophthalmol. 2008;36(1):8-12. 49. Sloan B, Scheinfeld, N. The use and safety of doxycycline hyclate and other second-generation tetracyclines. Expert Opin Drug Saf. 2008;7(5):571-7. 50. Yoo SE, Lee DC, Chang MH. The effect of low-dose doxycycline therapy in chronic meibomian gland dysfunction. Korean J Ophthalmol. 2005;19(4):258-63. |

