A 71-year-old black male presented to the Gainesville Veteran’s Affairs ophthalmology clinic complaining of vision loss in the inferior field of his left eye, which had persisted for two days. He stated that his vision was “dark at first, but now blurry—like looking through water.” He denied any pain, trauma or discharge from his left eye, and said that he did not have any flashes, floaters or a curtain coming into his vision. In addition, he denied any jaw claudication, scalp tenderness, fatigue or headaches that were consistent with giant cell arteritis.
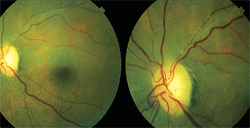
A fundus examination of the right and left eyes revealed Hollenhorst plaques causing blockage of the superior temporal artery with surrounding retinal edema.
The patient’s medical history was significant for hypertension, hyperlipidemia, cardiomyopathy, insulin-dependent diabetes mellitus, benign prostatic hyperplasia and deep vein thrombosis. His current medications included 0.125mg digoxin q.a.m. and 200mg metoprolol succinate q.d. for cardiomyopathy; 10mg glipizide b.i.d. and 100 units/mL insulin for type 2 insulin-dependent diabetes; 25mg hydrochlorothiazide/37.5mg triamterene q.d., 100mg losartan q.d., 40mg lisinopril q.d., and 30mg nifedipine t.i.d. for hypertension; 20mg rosuvastatin q.d. for hyperlipidemia; 2mg terazosin for benign prostatic hyperplasia; and 81mg aspirin q.d. for stroke and heart attack prevention.
His last eye exam was more than two years prior, and revealed mild non-proliferative diabetic retinopathy, mild hypertensive retinopathy, an epiretinal membrane and symptomatic dry eye syndrome in both eyes.
Diagnostic Data
Best-corrected visual acuity was 20/25 O.D. and 20/40- O.S. Confrontation fields were full in the right eye, but severely constricted in the inferior half of his left eye. Amsler grid testing was normal in the right eye, but revealed an inferior scotoma in the left eye. Anterior segment evaluation by slit lamp showed a few sebaceous cysts on both lower lids; quiet bulbar conjunctivae; mild corneal arcus without keratopathy in both eyes; deep and quiet anterior chambers without cells or flare and brown, flat and intact irides—without any rubeosis. On Goldmann applanation testing, his intraocular pressure (IOP) measured 14mm Hg O.U. We dilated the patient with one drop of phenylephrine 2.5% and one drop of tropicamide 1%.
We evaluated the posterior segment using a slit lamp, a 90D lens and binocular indirect ophthalmoscopy with a 20D lens. The crystalline lens showed 2+ nuclear sclerotic cataracts and 2+ anterior cortical cataracts O.U.
The fundus examination of the right eye revealed a clear vitreous, a 0.35/0.35 cup/disc ratio with good perfusion of the neuroretinal rim, and an artery/vein ratio of about 1/2 with mild arteriolar attenuation. The macula was flat, and the periphery was flat and intact with scattered areas of white without pressure.
The fundus examination of the left eye revealed a clear vitreous; a 0.45/0.45 cup/disc ratio with mild temporal pallor of the disc, an artery/vein ratio of 1/2 with mild arteriolar attenuation, and a sclerosed vessel, box-carring and refractile plaques emanating from the superior portion of the optic nerve head (figure 1). The macula of his left eye appeared diffusely pale and edematous superior to the fovea, and the periphery was flat and intact with scattered areas of white without pressure.
Differential Diagnoses
The differential diagnoses in this case included:
• Central retinal artery occlusion (CRAO), which usually manifests as superficial whitening of the retina in the posterior pole and a cherry red spot in the center of the macula. Visual acuity is normally finger counting or worse, with a marked afferent pupillary defect (APD).1
• Branch retinal artery occlusion (BRAO), which presents as a focal, wedge-shaped area of retinal whitening with a retinal emboli (or Hollenhorst plaque) visible in 62% of cases. Patients are usually in their seventh decade, and typically have hypertension, carotid occlusive disease and/or diabetes mellitus.1
• Ophthalmic artery occlusion, which presents with marked constriction of retinal vessels and marked retinal edema often without a cherry red spot. Visual acuity is severely reduced to the level of light perception or even no light perception.1
• Inflammatory or infectious retinitis, which may manifest as areas of retinal whitening or edema caused by a variety of infectious (herpes simplex virus, candidiasis, cytomegalovirus or toxoplasmosis) or inflammatory agents (Vogt-Koyanagi-Harada syndrome, sarcoidosis, serpiginous choroidopathy or Behçet’s disease).1,2
Diagnosis
Because the patient’s best-corrected visual acuity was 20/40 O.S. and there was no evidence of a cherry red spot in the macula, a diagnosis of CRAO or ophthalmic artery occlusion were excluded. Based on the findings of visible retinal emboli in the superior temporal arcade, a coinciding inferior altitudinal visual field defect and an area of retinal whitening within the distribution of a branch retinal arteriole, we diagnosed the patient with a BRAO O.S.
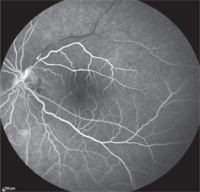
We performed spectral-domain optical coherence tomography (SD-OCT) and confirmed a large area of edema located just superior to the macula O.S. We then sent the patient directly to a retinologist for immediate evaluation. Intravenous fluorescein angiography (IVFA) showed normal perfusion in the right eye, and significantly delayed filling with an evident plaque in the superior arcade of his left eye (figure 2).
We alerted his primary care physician of the patient’s BRAO, and the retinologist recommended a complete cardiovascular evaluation, including an echocardiogram and carotid Doppler study.
We scheduled the patient to return to the retina clinic in one month for follow-up care. IVFA showed significantly delayed filling with an evident plaque located in the superior arcade of his left eye.
Follow-up Care
Visit 1
The patient returned to the retina clinic six weeks later. His best-corrected vision was 20/25- O.D. and 20/30-2 O.S. As measured by Tonopen (Reichert), IOP was 12mm Hg O.D. and 11mm Hg O.S.
Anterior segment was unremarkable, except for moderate nuclear sclerotic and cortical cataracts O.U. The irides were flat and intact, brown, and without rubeosis. Gonioscopy of the left eye revealed an open angle visible to the ciliary body band, mild iris processes and no evidence of neovascularization.
Dilated fundus exam of the left eye revealed a clear vitreous cavity, and a 0.45v/0.45h optic nerve with mild temporal pallor of the disc. The vessels looked attenuated with a severely sclerosed retinal artery emanating from the superior optic nerve head.
The macula appeared pale with about two disc diameters surrounding edema superior to the foveal avascular zone. The periphery was flat and intact, with scattered areas of white without pressure.
The patient was scheduled for a carotid Doppler test two weeks from the visit. Again, the retinal specialist recommended obtaining an echocardiogram. The patient was advised to control his blood pressure, blood sugar and cholesterol to prevent further complications. We scheduled him to return to the retina clinic in six weeks.
Visit 2
The patient returned to the ophthalmology clinic one month later complaining of a black-brownish floating spot he had in his left eye for four days. He denied the presence of any pain, flashes, curtain in his vision or new visual field loss. His best-corrected vision was 20/25- O.D. and 20/40+2 O.S. As measured by Tonopen, his IOP was 13mm Hg O.D. and 11mm Hg O.S. The anterior segment of the left eye was unremarkable except for the aforementioned cataracts, and there was no rubeosis present.
A dilated fundus exam of the left eye now revealed a 1/6 disc diameter preretinal hemorrhage located just inferior to the optic nerve head, with an adjacent retinal tuft and small particle vitreous hemorrhage. The optic nerve head now displayed some sectoral pallor inferiorly and temporally without shunt vessels present. The macula still appeared swollen just superiorly, and the periphery was flat and intact with no evidence of tears, breaks, holes or retinal detachment.
The carotid Doppler test completed two weeks prior revealed <49% stenosis of the right and left internal carotid arteries. The patient’s primary care physician was again alerted about the risk of mortality in patients with retinal artery occlusions, and further evaluation including echocardiogram and embolic work-up was recommended. We educated the patient on his exam findings and scheduled him to return to the retina clinic in one month.
The ophthalmologist recommended possible sectoral retinal photocoagulation if neovascularization became apparent once the vitreous hemorrhage resolved. We instructed the patient to return to the clinic immediately if he noticed any new floaters or changes in vision.
Visit 3
The patient returned to the ophthalmology clinic one month later for follow-up. He denied any changes in vision since the last visit. His best-corrected vision was 20/40 O.D. and O.S. Measured by Tonopen, his IOP was 14mm Hg O.D. and 15mm Hg O.S. The anterior segment of the left eye was unchanged.
A dilated fundus exam of the left eye now revealed possible neovascularization of the disc inferiorly, a cotton wool spot just nasal to the optic nerve head, and scattered dot and blot hemorrhages in the posterior pole. The macula still appeared swollen just superiorly, and the periphery was flat and intact with no evidence of tears, breaks, holes or retinal detachment.
We counseled the patient on the exam findings. The retinal specialist did not perform any retinal photocoagulation at this time. He wanted to evaluate the patient in two months to monitor for any worsening of the presumed neovascularization. We advised the patient to return to the clinic immediately if his symptoms changed.
Discussion
Clinical features
BRAOs are caused by a blockage in a branch of the central retinal artery leading to retinal ischemia in the affected area. They represent approximately 38% of all acute retinal artery obstructions.3 The main cause of this acute event is often an embolus that has traveled from another part of the body, becoming trapped in a vessel too narrow for passage.4 The point where the blockage occurs dictates the nomenclature (branch, twig, hemiretinal or central retinal artery occlusion). If the embolus were to become lodged at the lamina cribrosa, a central retinal artery occlusion would result.
Sometimes, however, the embolus is small enough to traverse the narrowing of the central retinal artery at the lamina cribrosa and become stuck in the smaller-caliber retinal arteries, resulting in a BRAO.4 Once this occurs, anoxia takes place in the inner two-thirds of the retina, including the nerve fiber layer, ganglion cell layer, inner plexiform layer and the inner portion of the inner nuclear layer. The outer third of the retina remains uncompromised, as its perfusion is supplied by the choroid. A study on rhesus monkeys proved that irreversible retinal necrosis occurs after 105 minutes of ischemia, but showed good recovery prior to 97 to 98 minutes of ischemia.5
Symptoms typically are sudden, unilateral and painless and include partial loss of vision. One study showed that ocular arterial occlusions can occur at any time; however, 65.1% of the surveyed population noticed visual deterioration during the daytime (between waking up and going to sleep).6 Patients often complain of a visual defect that corresponds with the site of the occlusion. A visual field test typically will show a superior or inferior altitudinal defect. Pupil examination may show an APD, depending on the size of the retinal infarction.
A careful case history may reveal that the patient had experienced previous episodes of amaurosis fugax, prior cerebrovascular accidents or other types of transient ischemic attacks. Visual acuity can range from 20/15 to finger counting, depending on the extent of macular involvement. The visual prognosis after a BRAO is favorable; one study found that 89% of eyes initially presenting with best-corrected visual acuity of 20/40 or better retained that vision after 14 months follow-up time.7
Depending on the timing, a dilated fundus exam of a fresh lesion will show a wedge-shaped area of superficial whitening within the zone affected by the BRAO.2 This phenomenon occurs due to ganglion cell necrosis, resulting in intracellular edema. Over several weeks, the retinal whitening resolves and the retina regains a relatively normal appearance. Although a rare finding, collateral blood vessels may form in the weeks and months following a vascular occlusion. These anomalous vessels represent an anastomosis between the obstructed arteriole and adjacent healthy arterioles in an attempt to re-perfuse the retina.8 BRAOs usually occur at bifurcations, most commonly in the superior temporal retina.9 Retinal or iris neovascularization is fairly uncommon, unless ocular ischemic conditions, such as diabetes mellitus or carotid occlusive disease, are present.1
Fundus fluorescein angiography will show delayed or absent filling in the affected branch, delayed arteriovenous transit time, reduced arterial caliber (attenuation) and segmental “box-carring” or “cattle-trucking” of the blood column.10 Also, the appearance of retrograde filling of the occluded branch retinal artery can help determine the prognosis of the vascular occlusion.11 Retrograde filling indicates a means of collateral circulation from the adjacent arterioles and capillaries.11 Despite this, retinal degeneration in the affected area still occurs because the blood supplied by the capillaries is incapable of delivering an adequate supply of oxygenated blood.12 Even though it is called a “branch retinal artery occlusion,” the circulation is often markedly delayed, but never totally interrupted.12
In addition to fluorescein angiography, noninvasive instruments such as OCT and SD-OCT can be used to histopathologically monitor changes in the retina. OCT scans of a BRAO show high reflectivity corresponding to the edematous inner retinal layer and a hyporeflective signal corresponding to the photoreceptor layer.13 The OCT can identify shrinkage and thinning (from neural cell loss) to a final thickness of 60% of a normal healthy retina.14 This finding can be used clinically, as a visual field defect can correspond to an atrophied retinal area despite a normal ophthalmoscopic appearance long after a BRAO has occurred.14
However, SD-OCT is much better in detecting pathological changes in the individual layers of the retina. It can be used to monitor changes in the foveal inner-segment-outer-segment line, which can be helpful in monitoring structural integrity of the photoreceptor layer despite atrophic changes in the inner retinal layers.13 Additionally, our Spectralis (Heidelberg Engineering) SD-OCT unit is equipped with TruTrack technology, which allows imaging of identical points on the retina at different time periods, with high correlation.13
Multifocal electroretinograms (MERGSs) can also identify damaged areas of the retina in patients with a BRAO. Both the first- and second-order MERGs are useful in evaluating retinal function; however, the second-order MERGs are more sensitive in detecting damage to the inner nuclear layer, inner plexiform layer, the ganglion cells and the nerve fiber layers.15 Although MERGs are not commonly used in all clinical settings, they are highly useful since a BRAO damages the inner two-thirds of the retina.
Etiology
By far, the most common cause of a BRAO is an embolus. The three most common types of emboli are cholesterol, calcific and platelet-fibrin.12 Other less common types include emboli from tumors, inflammation, bacteria, parasites, fungi, amniotic fluid or impurities injected into the bloodstream from intravenous drug use.12
The carotid artery and the heart are the two most common sources. In the carotid artery, emboli often originate from atheromatous disease (plaque). In the heart, emboli often come from aortic and mitral valvular lesions, tumors of the left atrium, myxomas or patent foramen ovales.16 Once the embolus dislodges from a vessel wall, it travels through the bloodstream until it reaches a site where it is too large to pass through. In this case, the emboli were small enough to pass through the lamina cribrosa and not cause a CRAO, but large enough to occlude a branch retinal artery.
The cholesterol embolus is the most common type of retinal embolus seen.17 These emboli often appear slightly larger than the blood vessel they are within, and are usually clumped as multiple tiny yellow crystals at the bifurcation.17 They often reflect brightly, depending on the angle of the light source. At times, they may not be visible ophthalmoscopically, but will shine a golden-orange with light digital pressure on the eye.18
A magnified view of the affected artery.
Cholesterol emboli will travel distally until they disappear from the branch retinal artery over the course of hours, days or weeks. Periarteriolar sheathing may be visible, indicating the earlier presence of a cholesterol embolus.18 When sheathing occurs at an arteriolar bifurcation, it is referred to as “pants leg sheathing,” which is pathognomonic of prior embolism.
Emboli are most frequently seen in the temporal retina by a 7:1 ratio, more so superiorly than inferiorly.17 Calcific emboli appear as solid, dirty yellowish-white colored lesions that do not shine with induced pressure. They have a tendency to become lodged in first- or second-order arteries, and often overlie the optic disc.18 They are larger than cholesterol emboli and more likely to occlude a vessel totally, remaining within the vessel forever. For that reason, they are more likely to produce a total or sectoral permanent loss of vision.
Platelet-fibrin plaques appear as dull, gray-white, mobile materials that tend to defragment as they travel throughout the vasculature. Also called “fisher plugs,” these emboli are difficult to observe due to their migratory behavior. Patients with platelet-fibrin plaques may be asymptomatic, may complain of amaurosis fugax or may have a BRAO.19 They presumably arise from ulcerative plaque of the ipsilateral internal carotid artery or from abnormal heart valves.
Laboratory Testing
Patients who present with either a CRAO or BRAO need an investigative workup to determine the underlying etiology. If the patient is 50 years or older, often the erythrocyte sedimentation rate (ESR) is ordered to rule out GCA. The clinician should also ask about cardinal symptoms of giant cell arteritis that include antecedent headache, jaw claudication, scalp tenderness, joint aches, recent weight loss or fever. Although the patient may deny any of those symptoms, the ESR should be ordered. Westergren ESR testing is the most reliable method, and the normal values for men are age divided by two and age plus 10 divided by two, for women. BRAO associated with GCA is relatively uncommon.
If the ESR is elevated or there is clinical suspicion of GCA, a temporal artery biopsy should be performed. Additionally, a C-reactive protein (an acute phase protein released in the bloodstream by the liver) is often ordered to determine the level of inflammation. A recent study reported that high levels of C-reactive protein correlate more with atherosclerosis and future risk of a life-threatening vascular event, and was not significantly elevated in patients with a retinal artery occlusion.20 The significance of increased C-reactive protein in patients with a retinal artery occlusion is probably due to risk factors such as essential hypertension, heart disease, hyperlipidemia and diabetes mellitus that can contribute to the vascular occlusive event.20
Systemic workup should also include checking blood pressure and pulse, carotid palpation and auscultation, fasting blood sugar, glycosylated hemoglobin, complete blood cell count with differential, and prothrombin time/partial thromboplastin time (PT/PTT). If the patient is less than age 50 or has appropriate risk factors, also consider a lipid profile, anti-nuclear antibody, rheumatoid factor, fluorescent treponemal antibody, serum protein electrophoresis, hemoglobin electrophoresis and anti-phospholipid antibodies.2
A carotid artery evaluation with Doppler ultrasonography and cardiac evaluation with echocardiography is warranted, because these patients likely have systemic comorbidities. Patients with both the presence of a visible retinal embolus and a BRAO have been shown to have a worsened survival prognosis.21 A recent study indicated that echocardiographic studies positively identified potential sources of emboli in the heart or aortic arch in 16 of 73 patients with retinal arterial occlusive events.22
Treatment and Management
Although there is not a single proven modality of treatment for a BRAO, there have been numerous hypothesized and attempted treatments aimed at increasing the perfusion pressure of the retinal circulation or dislodging the disrupting emboli. Retinal perfusion pressure may be increased by reducing the IOP, dilating the ophthalmic and central retinal arteries, or increasing the ophthalmic artery pressure.10 Oral acetazolamide (Diamox 500mg tablets, Barr Pharmaceuticals) can reduce the IOP as low as 5mm Hg very quickly, and has shown some benefit in the acute stage.
Anterior chamber paracentesis can also lower IOP dramatically; however, this modality is more controversial. A maximum increase in retinal perfusion of only 20% has been reported from animal studies; additionally, ophthalmologists may feel discouraged to perform this procedure due to the risk of complications and the need to repeat the paracentesis every two hours to maintain low IOP.10 Digital ocular massage, retrobulbar administration of vasodilating drugs and inhalation of carbon dioxide (from breathing into a paper bag or the premixed preparation carbogen) have been used to treat the acute stages of BRAO by activating the retinal auto-regulatory mechanisms.
The evidence shows that BRAOs are most often caused by emboli in patients with cardiovascular risk factors. Theoretically, visual recovery should be possible if started within the first few hours of the acute phase; however, most patients do not present in that narrow time period, and it is nearly impossible to recover ganglion cell loss after that critical period. Although there is still no proven treatment, optometrists should investigate for any carotid or cardiac conditions, because these patients have a significantly increased risk for stroke, heart attack or even death.
Dr. Cohen completed his residency at the Malcom Randall VA Medical Center in Gainesville, Fla., in June 2010. He now practices in a commercial setting in West Los Angeles. Dr. Marcus-Freeman is a staff optometrist and optometry residency coordinator at the Malcom Randall VA Medical Center.
1. Kaiser PK, Friedman NJ, Pineda R. The Massachusetts Eye and Ear Infirmary Illustrated Manual of Ophthalmology. 2nd ed. Philadelphia: Elsevier Publishing; 2004:229, 294-6.
2. Ehlers JP, Shah CP (eds). The Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2008:255.
3. Brown, GC, Shields JA. Cilioretinal arteries and retinal arterial occlusion. Arch Ophthalmol. 1979 Jan;97(1):84-92.
4. Alexander LJ. Primary Care of the Posterior Segment. 2nd ed. Stamford, CT: Appleton & Lange; 1994:327.
5. Hayreh SS, Weingeist TA. Experimental occlusion of the central retinal artery of the retina. IV: Retinal tolerance time to ischaemia. Br J Ophthalmol. 1980 Nov;64(11):818-25.
6. Schmidt D, Schumacher M, Feltgen N. Circadian incidence of non-inflammatory retinal artery occlusions. Graefes Arch Clin Exp Ophthalmol. 2009;247(4):491-4.
7. Mason JO 3rd, Shah AA, Vail RS, et al. Branch retinal artery occlusion: visual prognosis. Am J Ophthalmol. 2008 Sep;146(3):455-7.
8. Sharma MC, Volpe NJ. Collaterals in branch retinal artery occlusion. Ophthalmic Surg Lasers. 1999 Apr; 30(4):324-5.
9. Ros MA, Magargal LE, Uram M. Branch retinal-artery obstruction: a review of 201 eyes. Ann Ophthalmol. 1989 Mar; 21(3):103-7.
10. Beatty S, Au Eong KG. Acute occlusion of the retinal arteries: current concepts and recent advances in diagnosis and management. J Accid Emerg Med. 2000 Sep;17(5):324-9.
11. Schmidt D. A fluorescein angiographic study of branch retinal artery occlusion (BRAO) - the retrograde filling of occluded vessels. Eur J Med Res. 1999 Dec 16;4(12):491-506.
12. Rishard G, Soubrane G, Yannuzzi LA. Fluorescein and ICG Angiography: Textbook and Atlas. 2nd ed. New York, NY: Thieme Medical Publishers; 1998:66-70.
13. Murthy RK, Grover S, Chalam KV. Sequential spectral domain OCT documentation of retinal changes after branch retinal artery occlusion. Clin Ophthalmol. 2010 Apr 11;4:327-9.
14. Takahashi H, Iijima H. Sectoral thinning of the retina after branch retinal artery occlusion. Jpn J Ophthalmol. 2009 Sep;53(5):494-500.
15. Hasegawa S, Ohshima A, Hayakawa Y, et al. Multifocal electroretinograms in patients with branch retinal artery occlusion. Invest Ophthalmol Vis Sci. 2001 Jan;42(1):298-304.
16. Hayreh SS, Podhajsky PA, Zimmerman MB. Retinal artery occlusion: associated systemic and ophthalmic abnormalities. Ophthalmology. 2009 Oct;116(10):1928-36.
17. Madonna RJ. Optometry and the carotid artery. J Am Optom Assoc. 1993 Jun;64(6):390-402.
18. Younge BR. The significance of retinal emboli. J Clin Neuroophthalmol. 1989 Sep; 9(3):190-4.
19. Fisher CM. Observations of the fundus oculi in transient monocular blindness. Neurology. 1959 May; 9(5):333-47.
20. Goldenberg-Cohen N, Cohen Y, Monselise Y, et al. C-reactive protein levels do not correlate with retinal artery occlusion but with atherosclerosis. Eye (Lond). 2009;23(4):785-90.
21. De Potter P, Zografos L. Survival prognosis of patients with retinal artery occlusion and associated carotid artery disease. Graefes Arch Clin Exp Ophthalmol. 1993 Apr;231(4):212-6.
22. Mouradian M, Wijman CA, Tomasian D, et al. Echocardiographic findings of patients with retinal ischemia or embolism. J Neuroimaging. 2002 Jul;12(3):219-23.

