Ocular toxoplasmosis occurs as a consequence of Toxoplasma gondii infection. T. gondii, an obligate intracellular parasite, is estimated to infect at least one billion people worldwide.
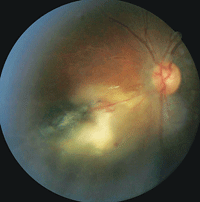
1. Fundus photograph of Patent 1’s left eye. Note the presence of a retinitis located adjacent to a large chorioretinal scar.
At least 25% of individuals who have T. gondii present with associated ocular manifestations.1 Ocular inflammation secondary to T. gondii infection is the most frequent cause of posterior uveitis.
Classic findings include a white fundus lesion with overlying, intense vitreous cells that frequently is described as “headlights in a fog.” The presentation of ocular toxoplasmosis can include a wide range of clinical signs, which poses a diagnostic challenge.
Here, we present three serologically confirmed cases of ocular toxoplasmosis and discuss the variable––and sometimes complex––clinical picture, including diagnostic and management strategies.
Patient 1
A 41-year-old black male presented with a chief complaint of floaters in his left eye that had persisted for three weeks. His medical history was remarkable for type 2 diabetes, which was controlled with oral medications.
His best-corrected visual acuity was 20/20 O.U. Intraocular pressure measured 12mm Hg O.D. and 15mm Hg O.S.
We identified several cells in the left vitreous. A dilated fundus examination revealed healthy optic nerves. Additionally, his right retina was unremarkable. In the left eye, however, we noted the presence of a retinitis that was located adjacent to a large chorioretinal scar (figure 1). We also detected a dilated retinal vein with associated superficial hemorrhages O.S.
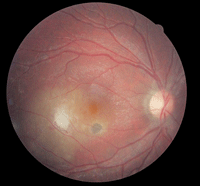
2. Fundus photograph of Patient 2’s right eye. What do you notice?
Patient 2
An 18-year-old white male presented with decreased vision in his right eye. His medical history was unremarkable, and his ocular history revealed possible evidence of an old infection O.D.
Best-corrected visual acuity was 20/50 O.D. and 20/20 O.S. His intraocular pressure measured 21mm Hg O.D. and 15mm Hg O.S. We documented panuveitis in his right eye.
Both optic nerve heads appeared healthy. However, there was an active retinochoroiditis with associated vitreous cells located temporal to the right fovea. Chorioretinal anastomosis was noted below the lesion. In addition, we identified a small chorioretinal scar that was associated with a neurosensory detachment located inferior to the right fovea (figure 2).
Patient 3
A 23-year-old white female presented with a painful left eye. She informed us that the pain began four days earlier, and was accompanied by photophobia. Her ocular and medical histories were unremarkable.
The anterior segment examination revealed several pigmented keratic precipitates, as well as cells and flare O.S. Intraocular pressure measured 18mm Hg O.D. and 24mm Hg O.S.
3, 4. On optical coherence tomography (below), Patient 3’s left eye exhibited a vitreous detachment. The fundus exam of his left eye (right) revealed the presence of a hemorrhagic retinal vasculitis as well as a chorioretinal scar.
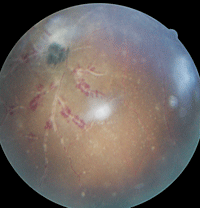

A dilated fundus examination of the right eye did not reveal any anomalous findings. However, her left eye exhibited a moderate amount of vitreous cells. On optical coherence tomography (OCT), we were able to further evaluate the vitreous cells as well as uncover the presence of an accompanying posterior vitreous detachment O.S. (figure 3).
Additionally, we documented an inferonasal midperipheral hemorrhagic vasculitis with an associated chorioretinal scar in her left eye (figure 4).
Discussion
• Overview of T. Gondii. The life cycle of T. gondii has multiple stages. Once the parasite is ingested by a cat (the primary host), it multiplies and is released into the feline feces. Once the intermediate host is exposed to the infected feces, the T. gondii oocysts rupture to release tachyzoites, which ultimately travel to target tissues and become bradyzoites.2
T. gondii infection can be acquired or congenital. Humans may acquire it by direct contact with infected feces (e.g., ingesting contaminated raw/undercooked meat and vegetables, consuming unpasteurized milk products or drinking contaminated water).
The risk of infection is significantly greater if the person has a compromised immune system (e.g., secondary to AIDS or chronic granulomatous disease), is of advanced age or is undergoing immunosuppressive therapy. Once infected, such high-risk individuals often experience a subsequent reactivation of a T. gondii infection. Also, it is important to note that there are rare case reports of patients becoming infected via blood transfusion or organ transplantation.3
In the congenital form, T. gondii is directly transferred through the placenta. Depending on when the fetus becomes infected, the disease may manifest with mild to severe signs and symptoms. Infection early in pregnancy typically leads to a more severe clinical presentation.
• Ocular Toxoplasmosis. Individuals with ocular toxoplasmosis may present with myriad signs and symptoms. These include decreased vision, floaters, pain or ocular redness.1-3 For example, Patient 1 complained of new floaters, while Patient 2 presented with a concern about decreased vision. On the other hand, Patient 3 presented with a painful, red eye.
The hallmark clinical finding of ocular toxoplasmosis is a retinochoroiditis. Characteristically, it appears as a fluffy, white or yellowish fundus lesion with overlying vitreous cells (Patient 2). The lesion initiates within the inner layers of the retina, while the choroid and sclera may become sites of contiguous inflammation and subsequent necrosis.4 The inflammation may lead to a posterior vitreous detachment (Patient 3) and/or the formation of vitreous “snowball” precipitates.
In addition, patients may have a granulomatous or non-granulomatous anterior uveitis (Patients 2 and 3). Severe inflammation may lead to posterior synechiae. Further, it is common to note increased intraocular pressure in patients who exhibit an accompanying anterior uveitis (Patients 2 and 3).
Inactive ocular toxoplasmosis fundus lesions appear as “punched-out” chorioretinal scarring. Upon reactivation of the initial infection, satellite areas of newly affected retinal tissue typically present adjacent to existing lesions.
Other posterior segment findings have been associated with ocular toxoplasmosis. Retinal vascular changes may include periphlebitis/arteritis (Patient 3), perivascular sheathing, branch retinal artery or vein occlusion (Patient 1), and choroidal anastomosis (Patient 2).5,6
Punctate outer retinal toxoplasmosis—characterized by multifocal punctate outer retinal lesions—is a less commonly reported finding that may be associated with a low-grade vitritis.7 Its appearance is similar to that of the white dot syndromes and histoplasmosis. (However, histoplasmosis does not present with an associated vitritis.7)
Complications from ocular toxoplasmosis may pose further diagnostic challenges as well as threaten the eye’s visual function. One such challenge is neovascularization secondary to retinal ischemia. Severe retinal vasculitis and/or inflammation, for example, may predispose a patient to neovascularization.8 The inflammatory response may extend to the optic nerve head, manifesting as an optic neuritis or papillitis.
Also, patients with ocular toxoplasmosis are at increased risk for retinochoroiditis juxtapapillaris (Jensen disease). Jensen disease has been described as an active retinal lesion with concomittant optic nerve involvement.9 Neuroretinitis in toxoplasmosis is associated with optic nerve head edema and macular exudate that presents in a stellate pattern (similar to the presentation exhibited by patients with cat scratch disease).
• Diagnosis and Treatment. A timely, accurate diagnosis of ocular toxoplasmosis is imperative to ensure that proper treatment is initiated immediately. In addition to a thorough clinical examinatinon, other diagnostic tests include OCT, ultrasonography, fluorescein angiography (FA) and indocyanine green angiography (ICG).
OCT is useful in determining the level of activity with respect to tissue depth, morphological changes and assocaited retinal edema, as well as aids in the evaluation of the vitreous and vitreoretinal layer. Also, OCT has shown that inflammation caused by T. gondii primarily involves the inner retina.
Other findings on OCT include epiretinal membrane, cystoid macular edema, vitreomacular traction, serous macular detachment and secondary choroidal neovascularization.10
FA can assist in the diagnosis of active toxoplasmosis lesions by depicting leakage and/or associated hypoperfusion. Further, ICG imaging may be used specifically to monitor for choroidal vascular activity.
The primary serologic test used to confirm the diagnosis is the Sabin-Feldman dye test. Additional laboratory evaluations include indirect fluorescent antibody testing as well as the enzyme-linked immunosorbent assay, IgG and IgM titers.
The clinical presentation of ocular toxoplasmosis may be similar to that associated with several other infectious and inflammatory conditions, including tuberculosis (TB), syphilis and sarcoidosis. In Patient 3, our differential diagnoses included TB, sarcoidosis and cytomegalovirus retinitis. Thus, a systemic evaluation (including serology) is necessary prior to the initiation of medical treatment. This is particularly imperative in cases with atypical presentations, because treatment with steroids may have a profound adverse effect.
Location, size and severity of the retinochoroiditis, as well as the systemic health of the patient, contribute to the overall management plan. In normal patients, peripheral lesions that present with minimal signs and symptoms simply may be monitored. However, if such signs and symptoms are evident in individuals with compromised immune systems, prompt treatment is warranted.
Further, lesions that are located in the posterior pole (Patients 1 and 2) are considered vision threatening. Thus, treatment is indicated for these lesions. Lesions that affect the macula or optic nerve, or those associated with two lines or more of vision loss, should receive treatment. Further, lesions that are associated with severe vitritis (Patient 3) also should be treated.
Conventional treatment is described as “triple” or “quad-ruple” therapy. The classic triple therapy consists of pyrimethamine, sulfadiazine and prednisone; quadruple therapy adds clindamycin as well. In patients with sulfa allergy, clindamycin is often substituted.
The typical treatment regimen persists for four to six weeks, with a high loading dosage during the first few days. Steroids are added two to three days after initiating the antibiotic therapy, and then are tapered during the next several weeks.
It is important to note that pyrimethamine use has been associated with adverse reactions and side effects, including thrombocytopenia and leukopenia.11 Thus, alternative antibiotics, such as azithromycin may be substituted. In most instances, however, folic acid supplementation simply is added to the drug regimen to avoid hematologic complications.
Contemporary treatment includes the use of Bactrim (sulfamethoxazole and trimethoprim, Mutual Pharmaceutical Company, Inc.), with adjunctive prednisone for a four- to six-week duration. This treatment option appears to be a safe and effective substitute for the conventional triple or quadruple therapeutic approaches.
A study showed that toxoplasmosis patients who use Bactrim are also 6.6% to 23.8% less likely to experience recurrent retinochoroiditis.11
Additional Discussion
During the last five years, researchers have identified several additional risk factors for T. gondii infection. Although it was once believed that most presentations of ocular toxoplasmosis were congenital in nature, the acquired form may be more common than previously considered. Higher dietary intake levels of locally cured, dried or smoked meat and unpasteurized goat’s milk, yogurt and cheese has been linked to increased parasite exposure.12 Eating raw oysters, clams or mussels also may increase an individual’s chances for ocular toxoplasmosis.12
The more time that passes without a reactivation following initial infection, the lower the patient’s overall probability of experiencing disease resurgence. However, epidemiologic data show that patients of advanced age have an increased risk of disease reactivation.13
Various studies regarding the epidemiology, prevalence and treatment of ocular toxoplasmosis are ongoing. For instance, one clinical trial is evaluating the treatment effect of intravitreal clindamycin plus dexamethasone.14 Preliminary data suggest that this drug combination may be a safer, more convenient treatment option than conventional triple or quadruple therapies.14
All three of our patients were managed successfully with either classic triple/quadruple therapy or the use of Bactrim and steroids for four weeks. Both treatment approaches yielded satisfactory disease resolution. More importantly, however, these cases illustrate the wide spectrum of ocular toxoplasmosis.
Atypical presentations can pose diagnostic dilemmas. Proper diagnosis can be established by carefully evaluating patient history, demographics and the overall clinical picture. Disease confirmation can be obtained serologically. It is critical to make a prompt, accurate diagnosis to ensure timely treatment and minimize or prevent secondary visual complications.
Dr. Reynolds is an associate professor at Nova Southeastern University School of Optometry in Ft. Lauderdale, Fla. Dr. Falco is an assistant professor at Nova Southeastern. Dr. Shechtman is an associate professor at Nova Southeastern and co-author of our “Research Review” column. Dr. Pizzimenti is an associate professor at Nova Southeastern and co-author of our “Review of Systems” column.
1. Vallochi AL, Nakamura MV, Schlesinger D, et al. Ocular toxoplasmosis: more than just what meets the eye. Scand J Immunol. 2002 Apr;55(4):324-8.
2. Perkins ES. Ocular toxoplasmosis. Br J Ophthalmol. 1973 Jan;57(1):1-17.
3. Da Mata AP, Orifice F. Toxoplasmosis. In: Foster CF, Vitale AT (eds). Diagnosis and Treatment of Uveitis. Philadelphia: Saunders; 2002.
4. Cordeiro CA, Moreira PR, Costa GC, et al. TNF-alpha gene polymorphism (-308G/A) and toxoplasmic retinochoroiditis. Br J Ophthalmol. 2008 Jul;92(7):986-8.
5. Küçükerdönmez C, Yilmaz G, Akova YA. Branch retinal arterial occlusion associated with toxoplasmic chorioretinitis. Ocul Immunol Inflamm. 2004 Sep;12(3):227-31.
6. Gentile RC, Berinstein DM, Oppenheim R, Walsh JB. Retinal vascular occlusion complicating acute toxoplasmosis retinochoroiditis. Can J Ophthalmol. 1997 Aug;32(5):354-8.
7. de Souza EC, Casella AM. Clinical and tomographic features of macular punctate outer retinal toxoplasmosis. Arch Ophthalmol. 2009 Oct;127(10):1390-4.
8. Benevento JD, Jager RD, Noble AG, et al. Toxoplasmosis-associated neovascular lesions treated successfully with ranibizumab and antiparasitic therapy. Arch Ophthalmol. Aug 2008;126(8):1152-6.
9. Eckert GU, Melamed J, Menegaz B. Optic nerve changes in ocular toxoplasmosis. Eye (Lond). 2007 Jun;21(6):746-51.
10. Monnet D. Optical coherence tomography in ocular toxoplasmosis. Int J Med Sci. 2009;6(3):137-8.
11. Commodaro AG, Belfort RN, Rizzo LV, et al. Ocular toxoplasmosis: an update and review of the literature. Mem Inst Oswaldo Cruz. 2009 Mar;104(2):345-50.
12. Jones JL, Dargelas V, Roberts J, et al. Risk factors for Toxoplasma gondii infection in the United States. Clin Infect Dis. 2009 Sep 15;49(6):878-84.
13. Holland GN, Crespi CM, ten Dam-van Loon N, et al. Analysis of recurrence patterns associated with toxoplasmic retinochoroiditis. Am J Ophthalmol. Jun 2008;145(6):1007-13.
14. Soheilian M, Ramezani A, Azimzadeh A, et al. Randomized trial of intravitreal clindamycin and dexamethasone versus pyrimethamine, sulfadiazine, and prednisolone in treatment of ocular toxoplasmosis. Ophthalmology. Jan 2011;118(1):134-41.

