Obesity is on the rise in the United States and worldwide. In turn, the incidence of idiopathic intracranial hypertension (IIH), also known as pseudotumor cerebri (PTC), is also rising.1 IIH initially presents as bilateral optic disc edema of unknown cause, and there are several etiologies, some of which are potentially deadly. Therefore, IIH is always a diagnosis of exclusion. This article reviews the presentation, diagnosis, testing and treatment of IIH.
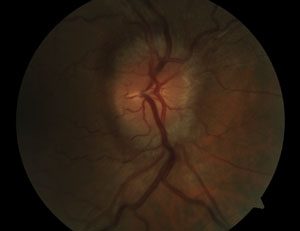 | |
| Fig. 1. Fundus photograph of a 52-year-old Hispanic male patient’s right eye. Note 360-degree disc edema with swelling of the adjacent retinal nerve fiber layer. Details of the major retinal arterial branches leaving the disc superonasally and inferiorly are obscured by the edema. |
Case Study
A 52-year-old Hispanic male presented with a chief complaint of episodic partial darkening of vision in his right eye that lasted five to 10 seconds, occurred up to 20 times a day and was associated with sudden lateral eye movement. He had first noticed this symptom eight months prior, but it had recently become more frequent. His medical history was remarkable for diabetes, hypertension and hyperlipidemia. His best-corrected visual acuity was 20/20-1 OD and 20/20-2 OS. Intraocular pressure measured 17mm Hg OD and 18mm Hg OS.
The right optic nerve exhibited 360-degree edema, which also involved the surrounding retinal nerve fiber layer (RNFL) (Figure 1). Subtle Paton’s lines (circumferential retinochoroidal folds) were also visible temporal to the disc. Retinal arterial branches leaving the disc were somewhat obscured by the edema in the right eye. The left optic nerve showed indistinct margins superonasally and inferiorly with apparent elevation of the superior and inferior neuroretinal rim (Figure 2). No major vessel obscuration was seen in the left eye. No hemorrhages, exudates or cotton wool spots were present. The retinal venules were not dilated or tortuous. He denied symptoms of diplopia, scalp tenderness, jaw claudication, new neck pain or headache and did not have pulseless, tender, enlarged or nodular temporal arteries upon palpation. Blood pressure measured 120/70mm Hg. His body mass index (BMI) was calculated to be 33.
This patient manifested a truly emergent ophthalmic condition: bilateral optic disc edema. Causes range from IIH to potentially fatal etiologies such as intracranial mass, dural venous sinus thrombosis and meningitis. We further investigated his bilateral disc edema with prompt additional testing, including baseline optical coherence tomography (OCT) (Figures 3, 4a and 4b), as well as same-day Humphrey visual field 30-2 SITA Fast testing.
The patient’s visual fields showed a superior defect in each eye with more prominent defect in the right eye and an enlarged blind spot in the right eye. We noted the patient had significant brow ptosis with dermatochalasis greater in the right eye than the left, and despite taping the lids the visual fields (Figures 5 and 6) still showed a residual superior defect in each eye, which we attributed to the patient’s very prominent overhanging brow. The enlarged blind spot present in the right eye could be correlated to the prominent disc edema noted in the right eye. Color vision with Ishihara pseudo-isochromatic plates was normal and cranial nerve testing did not reveal any other abnormalities.
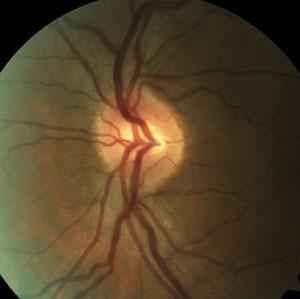 | |
| Fig 2. Fundus photo of the patient’s left eye. Note the more subtle findings than in the right eye, with slightly indistinct superior and inferior disc margins. |
History and Imaging
We thoroughly reviewed his medications and medical history and did not discover any potentially causative medication or systemic disease associated with intracranial hypertension. Even if you discover associated diseases or potentially causative medications, other life-threatening causes of bilateral disc edema must be ruled out.
We educated our patient about the emergent nature of bilateral disc edema and directly contacted the nearest emergency room with instructions to perform same-day MRI of the brain and optic nerves with contrast, plus magnetic resonance venography (MRV) of the brain.
We requested that, in the event the results of neuroimaging were unremarkable, lumbar puncture (LP) be performed with opening pressure recorded and cerebrospinal fluid (CSF) sent for biochemistry, microbiology and cytology. MRI and MRV were unremarkable. Opening pressure with LP was elevated and recorded at 26cm H20. CSF biochemistry, microbiology and cytology were normal.
After exclusion of potentially life-threatening conditions and following normal examination by a general neurologist, a diagnosis of IIH was established.
IIH Presentation
The overall annual incidence of IIH is between one and two in 100,000 in the general population.1,2 Among young, obese women, the estimated incidence is between 12 and 20 in 100,000.1,2
| Differential Diagnosis of Bilateral Disc Edema • Tumor • Intracranial bleed • Hydrocephalus • Aquaductal stenosis • Venous sinus thrombosis • IIH |
Symptoms of IIH are varied. The most common presenting symptom is headache, occurring in more than 90% of patients.1,2 Also common are transient visual obscurations, described as monocular or binocular blurring lasting a few seconds, thought to represent momentary ischemia of the already compressed optic nerve microvasculature.1 Many patients will complain of hearing something resembling a heartbeat in their ear, called pulsatile tinnitus. Other common complaints are photopsia and retrobulbar pain. Some patients may have diplopia related to an accompanying cranial nerve (CN) VI palsy (or rarely an associated CN III or IV palsy).1-3 In some instances there may be no reported symptoms, with the only sign being the presence of disc swelling.4 Our patient had some atypical characteristics for IIH being older and male, but studies have shown that both older patients and male patients with IIH had fewer complaints of headaches and were more likely to complain of visual disturbances.5
The hallmark sign of IIH is optic disc swelling (papilledema). Papilledema is caused by increased intracranial pressure.1,4 Disc swelling of unclear cause should be labeled as disc edema and should not be categorized as papilledema until it is confirmed with an elevated intracranial pressure reading.4 The disc swelling in papilledema is almost always bilateral, although it may be very asymmetric, making it appear unilateral.1,4 In papilledema, the elevated CSF pressure disturbs the normal gradient between intraocular pressure and retrolaminar pressure, resulting in increased tissue pressure within the optic nerve. This interferes with axoplasmic flow and produces neuronal flow stasis.6 Under this resistance the constipated axons swell, producing congestion at the level of the optic disc.7 Along with the general congestion, resultant optic nerve ischemia, orbital venous stasis and collateral impact on optic pathways resulting from adjacent dilated ventricles may play a role in producing variable visual sequelae.6,8
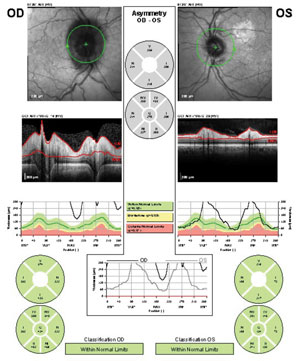 | |
| Fig. 3. Our patient’s OCT RNFL results. Note the off-the-chart RNFL edema in the right eye and the pronounced edema superior and inferior in the left eye (compare to the subtle superior and inferior edema in the photograph of the same eye in Figure 2). |
Obesity has long been reported to be the most commonly associated risk factor in IIH.9 Studies indicate between 74% and 94% of patients diagnosed with IIH are obese.5 Generally, obesity is considered to be a body mass index (BMI) greater than 30. Many patients who are not necessarily obese may be categorized as overweight. Some patients with IIH actually have normal BMI. However, in a recent study of 407 adult patients with IIH, only 4% had normal BMI, and of those, more than a quarter were reportedly exposed to medications known to be associated with IIH.5 Thus, care should be taken to uncover potential alternative causes of IIH in patients with normal BMI.5
Also, both obese and non-obese patients with a recent weight gain of 5% to 15% are at greater risk for developing IIH.9
The pathophysiology to explain the exact role of obesity in IIH remains unknown. Hypotheses have included metabolic and hormonal dysregulation. Also, obesity has been suggested to raise intra-abdominal pressure, in turn increasing pleural and cardiac filling pressure leading to increased intracranial venous pressure and thus elevated intracranial pressure.9 Our patient was atypical in that he was older and male but typical for IIH in that his BMI was >30, which categorizes him as obese; however obesity alone does not make the diagnosis.
Diagnostic Criteria
The basis for the diagnosis of IIH is the modified Dandy criteria (Table 1).1,10,11 The first step in diagnosing IIH is to obtain same-day MRI of the brain and optic nerves with contrast, plus MRV of the brain in order to rule out life-threatening causes of bilateral disc edema. Compressive tumors or hemorrhages can be visualized with MRI. Large space-occupying lesions can directly cause bilateral disc edema, while smaller brain tumors may cause obstructive hydrocephalus.4 This occurs when CSF is blocked along the narrow passages connecting the ventricles of the brain, which is detectable on MRI showing dilation of one or more of the ventricles in the brain.4,12 A specific form of obstructive hydrocephalus called aqueductal stenosis can also be detected with MRI.
MRV is needed to visualize a dural venous sinus thrombosis that may be missed with MRI alone. Because MRI and MRV can both miss a communicating hydrocephalus, which occurs when CSF is blocked after exiting the ventricles of the brain, LP is always indicated if MRI and MRV are normal.4,12 LP with CSF sent for biochemistry, microbiology and cytology is necessary to reveal infectious or carcinomatous meningitis, which are potential causes of a communicating hydrocephalus.4
| Table 1. Modified Dandy Criteria1,10,11 1. Presence of signs and symptoms of increased intracranial pressure. 2. Absence of localized findings on neurologic examination except those known to occur from increased intracranial pressure. 3. Absence of deformity, displacement or obstruction of the ventricular system and otherwise normal neurodiagnostic studies, except for evidence of increased cerebrospinal fluid pressure (>20cm H2O). 4. Abnormal neuroimaging except for empty sella turcica, optic nerve sheath with filled out CSF spaces and smooth-walled non flow-related venous sinus stenosis or collapse should lead to another diagnosis. 5. Awake and alert patient. 6. No other cause of increased intracranial pressure present. |
If CSF is normal and opening pressure on LP is elevated (>20cm H20), the modified Dandy criteria are still not met. The next step is a neurology referral to rule out localized findings with the exception of those known to occur with increased intracranial pressure such as CN VI palsy. The final diagnostic criteria mandate identifying all other potential causes of increased intracranial pressure. One cause of IIH is excess vitamin A consumption via use of some acne medications or diet supplementation.1,4,13 Other medications, such as tetracycline, corticosteroids and systemic conditions—e.g., obstructive sleep apnea, renal failure and severe anemia—are associated with IIH.4
OCT and Ophthalmoscopy
Although ophthalmoscopy remains the most valuable and critical first-level investigation, OCT can be a useful tool for differentiation between disc edema and pseudo disc edema. Pseudoedema may be present in patients with optic disc drusen, which has been found in approximately 2% of adult autopsy eyes.14 Optic disc drusen can alter the presentation of the optic disc, making it appear swollen. B-scan ultrasonography and fundus autofluorescence can be helpful to identify optic nerve drusen as well. With OCT, disc drusen are revealed with a “lumpy, bumpy internal contour,” whereas true optic disc edema is recorded with a “smooth internal contour.”14,15 Our patient’s OCT revealed a smooth internal contour consistent with true disc edema (Figures 4a and 4b). Other causes of pseudopapilledema include tilted optic disc, myelinated nerve fibers, congenitally crowded discs and optic nerve hypoplasia. Investigators found with OCT that the mean RNFL thickness is significantly greater in patients with true disc edema compared with those with pseudoedema of any type.15 In fact, in cases of pseudopapilledema, RNFL thickness tends to be normal in all four quadrants.16
Ophthalmoscopy can also be used to distinguish true edema from pseudoedema. One study found that the presence of disc edema with associated chorioretinal folds (Paton’s lines) had 100% sensitivity, and this finding is pathognomonic for true optic disc edema.14 Unfortunately, these folds were only present in 23% of patients in the study. Of the signs evaluated, swelling of the peripapillary RNFL had the highest accuracy as a single sign.14 Our patient had Paton’s lines associated with the edema of the right optic disc, indicating true disc edema (Figure 1).
| Table 2. Modified Frisén Scale17 | |||
| Edema Grade | Degree of Edema | Description of Findings | |
| 0 | Normal | Prominence of the retinal nerve fiber layer at the nasal, superior, and inferior poles in inverse proportion to disc diameter. Radial nerve fiber layer striations, without tortuosity. | |
| 1 | Minimal | C-shaped halo that is subtle and grayish with a temporal gap and obscures underlying retinal details.* Disruption of normal radial nerve fiber layer arrangement striations. Temporal disc margin normal. | |
| 2 | Low | Circumferential halo.* Elevation (nasal border). No major vessel obscuration. | |
| 3 | Moderate | Obscuration of > or = one segment of major blood vessels leaving disc.* Circumferential halo. Elevation (all borders). Halo (irregular outer fringe with finger-like extensions). | |
| 4 | Marked | Total obscuration of a segment of a major blood vessel on the disc*. Elevation (whole nerve head, including the cup). Border obscuration (complete). Halo (complete). | |
| 5 | Severe | Obscuration of all vessels on the disc and leaving the disc.* | |
| *Key features (major findings) for each grade | |||
The standard method of grading disc edema by ophthalmoscopy, the Modified Frisén Scale, is an ordinal scale with subjective evaluation and grades ranging from zero (no edema) to five (severe degree of edema) with characteristic findings for each increased grade (Table 2).17
As opposed to Frisén grading, OCT offers an objective and continuous scale for measurement of edema and has been shown to be helpful to confirm the presence of edema, especially in mild cases.18 The recently concluded Idiopathic Intracranial Hypertension Treatment Trial (IIHTT) found a strong correlation between OCT of papilledema at baseline and measures of swelling at the optic nerve head (ONH) with Frisén grading of papilledema at baseline.18 The data in this OCT sub-study of the IIHTT is being evaluated to see whether OCT parameters are superior to Frisén grading in monitoring papilledema resolution. The investigators are also evaluating whether OCT findings can be correlated to visual outcome.18
In addition to the measurement of peripapillary RNFL thickness with OCT, it has been suggested that total ONH volume and ONH height may also be useful data points for diagnosing and monitoring disc edema.15,19 OCT offers a non-invasive method to confirm disc edema in initial diagnosis and serves as an adjunct to ophthalmoscopy in following the course of the disease throughout treatment.
Don’t Forget Perimetry
Both OCT and automated perimetry should be used in combination to follow patients with papilledema.3 Visual field defects can range from absent to severe. During baseline visual field testing in the IIHTT researchers discovered that nerve fiber bundle-like defects, similar to those in glaucoma, are characteristic of optic nerve damage from increased intracranial pressure. This type of defect was present in 60% of patients at baseline testing in the IIHTT. The most common specific visual field defect found in this study was a partial arcuate scotoma combined with an enlarged blind spot. Diffuse visual field loss or neurologic-like defects were not commonly found.6
Medications and Weight Loss
The standard first-line medical treatment in IIH is oral acetazolamide. Until the IIHTT, no properly designed clinical trials had been performed to provide an evidence base for acetazolamide treatment. In the study, patients diagnosed with IIH were treated with either acetazolamide combined with a low sodium diet for weight reduction or placebo combined with the same low sodium diet.18
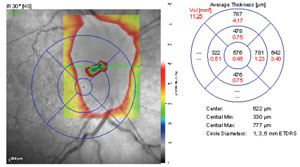 | ||
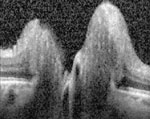 | Figs. 4a and 4b. Spectralis OCT optic nerve head thickness map of our patient’s right eye. Although the thickness map is not centered directly over the nerve on this scan, the raster line is directed through the optic nerve head, revealing temporal and nasal edema with smooth internal contour. | |
The acetazolamide group was reported to have decreased papilledema, increased vision-related quality of life and showed a modest improvement in visual field function compared with the placebo group.18 Acetazolamide is thought to work by inhibiting carbonic anhydrase causing a reduction in transport of sodium ions across the choroid plexus epithelium where CSF is produced in the brain. Research shows it can decrease CSF production in humans by anywhere from 6% to 50%.10
Neurologists and neuro-ophthalmologists treating IIH may pursue alternatives to acetazolamide if it is not tolerated. The most frequent early side effects with acetazolamide use are tingling in the fingers, toes and perioral region, called parasthesias. Other common symptoms include gastrointestinal upset, diuresis and malaise.20 Second-line medical therapy is usually with an oral diuretic like furosemide. Sometimes topiramate is used.
All medical therapies should be combined with a weight loss plan in obese and overweight patients to ultimately resolve the papilledema and preserve visual function. Normal BMI in IIH is rare, and in a recent study, none of the patients with IIH and normal BMI had severe visual loss in either eye. They were only treated medically, since weight loss was not needed for these patients.5 Weight loss is the key to long-term success in the vast majority of patients treated for IIH. Clinical improvement in IIH has been associated with as little as 6% weight loss.21 However, one study found a relapse rate of 28% in those treated for IIH and the relapse was associated with recent significant weight gain compared with non-relapsed patients.2 Ultimately, some patients may be offered gastric bypass surgery for significant weight loss and long-term resolution of papilledema in IIH.1
Surgical Intervention
In patients with sudden and severe papilledema who may be at risk for irreversible vision loss, surgical intervention may be urgently needed. This form of IIH (called fulminant IIH) is rare, but potentially devastating. Blindness is reported in up to 10% of these patients, and visual field defects of some type occur in up to 90%.21 Optic nerve sheath fenestration (ONSF) and cerebrospinal fluid shunting (CSFS) are the most commonly used surgical procedures, but it remains unclear whether one procedure is superior to the other.1
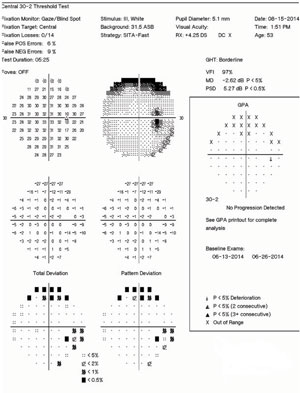 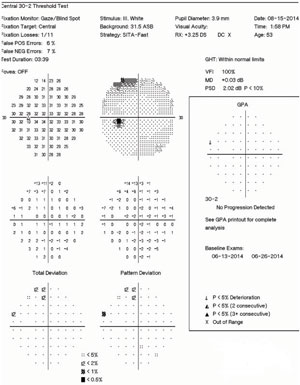 |
| Figs. 5 and 6. At right, our patient’s right eye HVF 30-2 SITA Fast results. The superior defect likely represents a brow artifact, but the clustered inferior temporal points reveal an enlarged blind spot. At left, his left eye HVF 30-2 SITA Fast results. The mildly depressed points superiorly likely represent interference of the patient’s brow. |
ONSF improves, or at least stabilizes, vision in 70% to 90% of cases and it has a lower complication rate compared with CSFS.1,22 However, headache symptoms significantly improved in only 31% of cases with ONSF.1 CSFS (by either ventriculoperitoneal or lumboperitoneal shunt) fail and require revision in up to 50% of cases.1 However, one series showed 95% of patients reported significant improvement in their headache symptoms after shunting was completed.1
Investigators have proposed that in patients where vision is threatened and headaches are not severe, ONSF is the procedure of choice, and in cases with severe headaches, regardless of visual status, CSFS may be the preferred procedure after failure of medical therapy combined with weight loss.4
Our patient is currently medically managed by a neurologist. He is also followed at regular intervals in our eye clinic with dilated fundus evaluation, optic disc photos, OCT of the optic nerve and nerve fiber layer and automated threshold perimetry. Our patient’s BMI classifies him as obese. We educated him on the value of weight loss in the management of IIH and referred him to a dietician. He was initially medicated with acetazolamide, and the enlarged blind spot on the visual field of his right eye has since resolved.
Although visual outcomes may be favorable with weight loss and medication, relapses with weight gain and the fact that optic nerve atrophy has been detected in well-treated patients suggest that long-term follow-up of IIH is necessary.2 Regular fundus examination with repeated OCT and automated threshold perimetry over many years should be performed along with comanagement with a neurologist or neuro-ophthalmologist.
Eye care professionals may be the first to detect IIH, as we perform ophthalmoscopy on a routine basis. Bilateral optic disc edema is a true ocular and medical emergency, even in patients without symptoms who are 20/20 and have a normal visual field. Life-threatening conditions must be urgently ruled out, as IIH is a diagnosis of exclusion.
Drs. Lynne and Walker are on staff at the Orlando VA Medical Center in Florida. Dr. Pizzimenti is an associate professor at Nova Southeastern University College of Optometry in Fort Lauderdale, Fla.
1. Wakerley B, Tan M, Ting Y. Idiopathic intracranial hypertension. Cephalalgia. 2015;35(3):248-61.2. Yri H, Wegener M, Sander B, Jensen R. Idiopathic intracranial hypertension is not benign: a long-term outcome study. J Neurol. 2012;259(5):886-94.
3. Skau M, Sander B, Milea D, Jensen R. Disease activity in idiopathic intracranial hypertension: a 3-month follow-up study. J Neurol. 2011;258(2):277-83.
4. Pane A, Miller N, Burdon M et al. The neuro-ophthalmology survival guide. Mosby; 2007.
5. Bruce B, Kedar S, VanStavern G, et al. Atypical idiopathic intracranial hypertension: normal BMI and older patients. Neurology. 2010;74(22):1827-32.
6. Keltner J, Johnson C, Cello K, et al. Baseline visual field findings in the Idiopathic Intracranial Hypertension Treatment Trial (IIHTT). Invest Ophthalmol Vis Sci. 2014;55(5):3200-7.
7. Skau M, Yri H, Sander B, et al. Diagnostic value of optical coherence tomography for intracranial pressure in idiopathic intracranial hypertension. Graefes Arch Clin Exp Ophthalmol. 2013;251(2):567-74.
8. Agarwal M, Yoo J. Optic nerve sheath fenestration for vision preservation in idiopathic intracranial hypertension. Neurosurg Focus. 2007;23(5):E7.
9. Almarzouqi S, Morgan M, Lee A. Idiopathic intracranial hypertension in the Middle East: A growing concern. Saudi J Ophthalmol. 2015;29(1):26-31.
10. Wall M, McDermott M, Kieburtz K, et al. Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss: the idiopathic intracranial hypertension treatment trial. JAMA. 2014;311(16):1641-51.
11. Waisbourd M, Leibovitch I, Goldenberg D, Kesler A. OCT assessment of morphological changes of the optic nerve head and macula in idiopathic intracranial hypertension. Clin Neurol Neurosurg. 2011;113(10):839-43.
12. National Institute of Neurological Disorders and Stroke Hydrocephalus Fact Sheet. (n.d.). In National Institutes of Health. Retrieved from http://www.ninds.nih.gov/.
13. Benzimra J, Simon S, Sinclair A, Mollan S. Sight-threatening pseudotumour cerebri associated with excess vitamin A supplementation. Practical Neurology. 2014;15(1):72-3.
14. Carta A, Favilla S, Prato M, et al. Accuracy of funduscopy to identify true edema versus pseudoedema of the optic disc. Invest Ophthalmol Vis Sci. 2012;53(1):1-6.
15. Heidary G, Rizzo J. Use of optical coherence tomography to evaluate papilledema and pseudopapilledema. Semin Ophthalmol. 2010;25(5-6):198-205.
16. Bassi S, Mohana K. Optical coherence tomography in papilledema and pseudopapilledema with and without optic nerve head drusen. Indian J Ophthalmol. 2014;62(12):1146-51.
17. Scott C, Kardon R, Lee A, et al. Diagnosis and grading of papilledema in patients with raised intracranial pressure using optical coherence tomography vs clinical expert assessment using a clinical staging scale. Arch Ophthalmol. 2010;128(6):705-11.
18. Auinger P, Durbin M, Feldon S, et al. Baseline OCT measurements in the idiopathic intracranial hypertension treatment trial, part II: correlations and relationship to clinical features. Invest Ophthalmol Vis Sci. 2014;55(12):8173-9.
19. Auinger P, Durbin M, Feldon S, et al. Baseline OCT measurements in the idiopathic intracranial hypertension treatment trial, part I: quality control, comparisons, and variability. Invest Ophthalmol Vis Sci. 2014;55(12):8180-8.
20. Kassamali R, Sica DA. Acetazolamide: a forgotten diuretic agent. Cardiol Rev. 2011 Nov-Dec;19(6):276-8.
21. Johnson L, Krohel G, Madsen R, March G. The role of weight loss and acetazolamide in the treatment of idiopathic intracranial hypertension (pseudotumor cerebri). Ophthalmology. 1998;105(12):2313-7.
22. Alsuhaibani A, Carter K, Nerad J, Lee A. Effect of optic nerve sheath fenestration on papilledema of the operated and the contralateral nonoperated eyes in idiopathic intracranial hypertension. Ophthalmology. 2011;118(2):412-4.

