 |
History
A 62-year-old white female presented for a routine eye examination with a chief complaint of progressively decreasing vision in her left eye during the past two months.
Her ocular and systemic histories were unremarkable. She denied taking medication of any kind.
Diagnostic Data
Best-corrected visual acuity was 20/20 O.D. and 20/60 O.S. External examination revealed a central distortion upon facial Amsler testing in her left eye; this was substantiated with the formal grid. The remaining external testing data were normal, and there was no evidence of afferent pupillary defect.
Slit lamp exam revealed healthy anterior segments in both eyes. Applanation tonometry measured IOPs of 16mm Hg O.D. and 18mm Hg O.S.
The photograph demonstrates the pertinent retinal findings.
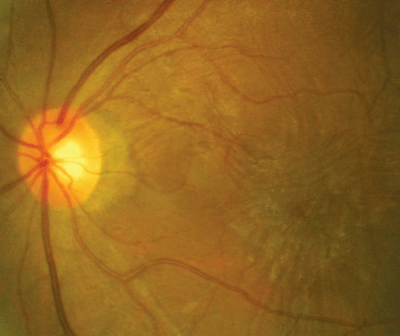 |
| A 62-year-old female presented with decreasing vision and this retinal appearance. |
How would you approach this case? Does this patient require additional tests? What is your diagnosis? How would you manage this patient? What is the likely prognosis?
Discussion
The diagnosis in this case is an epiretinal membrane (ERM) in the patient"s left eye. An epiretinal membrane is a fibrocellular, avascular membrane that proliferates on the internal limiting membrane of the retina. This, in turn, produces a varying degree of macular dysfunction.1
Ancillary testing that can aid in the diagnosis includes visual acuity measurement, Amsler grid testing, contact lens biomicroscopy, the Watzke/Allen test for macular hole formation (projection of a solid line onto the macula to see if the patient perceives it as broken or interrupted), red-free photography and fluorescein angiography.1
ERMs typically occur in patients ages 50 and older, with increased incidence with advancing age.1-3 Most ERMs are idiopathic, although they can occur secondary to numerous ocular pathologies.1-3 Risk factors include any retinal surgery, preoperative vitreous hemorrhage, macular detachment, large retinal breaks and preoperative proliferative vitreoretinopathy.1
ERMs are commonly associated with blunt or penetrating trauma, vitreous inflammation, vitreous hemorrhage and chronic retinal edema secondary to any retinal vascular disease.1
One hypothesis suggests that ERMs result from migration of retinal glial cells through a defect in the internal limiting membrane (ILM).1 In idiopathic cases, the defect in the ILM is likely secondary to vitreous dehiscence from the ILM during a posterior vitreous detachment.1 ERMs that develop in eyes that have retinal breaks often form with proliferative vitreoretinopathy.1 One study found that an ERM is not composed of a single cell type but multiple cellular entities; of these, no one cell type could be identified as the origin.4
ERMs are typically classified according to the density and contractile properties they exhibit.2,3 The three classifications:
Cellophane maculopathy. This term connotes a thin, glistening, almost transparent membrane.1,3
Surface wrinkling maculopathy. This disturbance occurs as the ERM begins to contract, producing traction on and around the macula. This causes the macula to appear wrinkled, most notably in the outermost margins.1,3
Macular pucker. This end-stage progression occurs as the ERM contracts. Increased thickening with almost a grayish-white appearance ensues with accompanied pronounced wrinkling.1,3 Here, forces are sufficient to induce tractional macular detachments, retinal hemorrhages and the formation of a macular hole.1
Depending on the stage, patients may be asymptomatic, or they may present with metamorphopsia or frank vision loss.1 Differential diagnoses include cystoid macular edema, proliferative vitreoretinopathy, prominent macular reflex with plush nerve fiber layer, optic disc swelling with Paton"s lines, combined retinal pigment epithelium, retinal hamartoma or idiopathic macular hole.1
An ERM in which the patient is asymptomatic requires only monitoring.1 Careful, regular, in-office follow-up observation, documentation and home monitoring with a take-home Amsler grid are reasonable methods of charting stability.
Consider referring patients whose membranes produce visual loss for further retinal evaluation to rule out a vitrectomy and membrane peel with gas tamponade and face-down positioning.1,2,5 Patients whose vision loss is less than 20/60 have the best prognosis.1,5 Patients who have acuity of 20/60 to 20/100 may regain slightly more vision postoperatively than patients who have acuity of 20/100 or worse when they undergo the procedure. However, patients whose vision is poorer than 20/100 tend to perceive the greatest amounts of improvement.5
Thanks to Steve Sheehan, O.D. for contributing this case.
1. Johnson MW. Epiretinal Membrane. In: Yanoff M, Duker JS. Ophthalmology. Philadelphia: Mosby; 1999:8.32.1-8.32.4.
2. Pournaras CJ, Donati G, Brazitikos PD, et al. Macular epiretinal membranes. Semin Ophthalmol 2000 Jun;15(2):100-7.
3. Jacobsen CH. Epiretinal membranes. Optom Clin 1996;5(1):77-94.
4. MacDonald IM, Smith CH, Britton WA Jr. Cell culture of macular epiretinal membranes. Can J Ophthalmol 1992 Dec;27(7):331-5.
5. Rice TA, De Bustros S, Michels RG, et al. Prognostic factors in vitrectomy for epiretinal membranes of the macula. Ophthalmology 1986 May;93(5):602-10.

