26th Annual Surgery Report- Pearls of Postoperative Care- MIGS: Indications and Complications |
With near-instantaneous, life-changing visual improvement, laser-assisted in situ keratomileusis (LASIK) is widely considered one of the best elective procedures, with postoperative dissatisfaction rates near 1%.1 Another marker of post-op success is visual acuity, and PROWL 1 and 2, which detailed patient-reported outcomes with LASIK, found uncorrected visual acuity (UCVA) of 20/20 or better in 97.5% and 91.5% of patients, respectively.2,3
It’s no wonder LASIK is a mainstay for patients with ametropia who desire optical independence. But LASIK isn’t the only game in town now, and patients have some choices to make when deciding on the best refractive surgery option for them. Here, we discuss where LASIK currently stands and some of the newer procedures available, including small-incision lenticule extraction (SMILE), implantable collamer lens (ICL) and refractive lens exchange (RLE).
 |
|
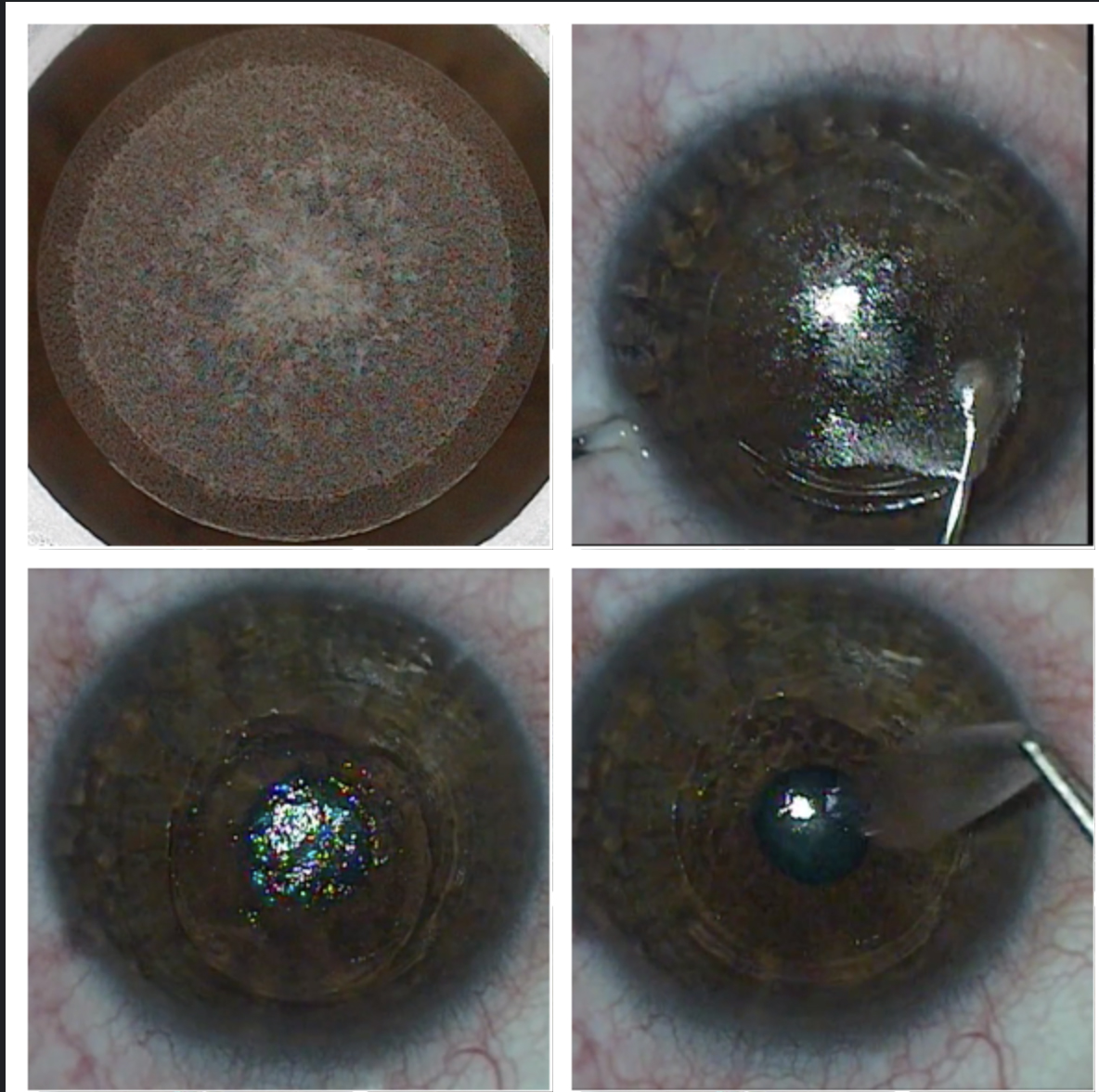
The SMILE procedure creates (top left), dissects (top right) and removes a lenticule (bottom left and right) to correct myopia and myopic astigmatism. Click image to enlarge. |
LASIK Basics
This surgical option involves two major steps: (1) the creation of a stromal flap and (2) laser tissue ablation. In corneal refractive surgery, LASIK is unique in that it necessitates two lasers—a femtosecond laser for flap creation and an excimer laser to reshape the corneal curvature. Flap creation significantly accelerates visual recovery, which lends itself to near-instant gratification for patients. Postoperatively, patients typically experience transient symptoms, such as mild stinging, watering and photophobia.
LASIK candidacy covers a wide patient base. The procedure is FDA-approved for myopia up to 14.00D and hyperopia and astigmatism up to 6.00D.4 However, in our clinic we typically only treat up to 10.00D of myopia, 4.00D of hyperopia and 6.00D of astigmatism. If a patient fits within these parameters, the first consideration is corneal thickness, which needs to be sufficient to safely accommodate the necessary dioptric corrections. Average laser flaps are around 110µm, and we know the procedure removes around 16µm of tissue per diopter. These numbers help surgeons calculate residual stromal bed (RSB). A common conservative RSB is 300µm with more aggressive surgeons approaching 250µm. Clinicians should rule out mechanical eye-rubbing and measure corneal biometry to better understand when surgery might leave a thinner RSB.
Other pre-op parameters include corneal curvature, corneal sensitivity and, to some degree, pupil size.
For refractive stability, clinicians should wait to perform LASIK until age 18 at the earliest. LASIK has no upper age limit, and many patients who undergo refractive cataract surgery benefit from a postoperative LASIK enhancement.
LASIK Advances | |||
| Technology | Process | Benefits | |
| Wavefront-guided | Wavefront aberrometers pass a single beam of light through the tear film, cornea, lens and vitreous, and the image that reports back from the retina is a patient’s wavefront. | This measures lower-order aberrations, such as sphere and cylinder, and HOAs, such as coma, trefoil and spherical aberration. This is a great choice for patients with high preexisting HOAs. | |
Wavefront-optimized | This theoretically bypasses the individual’s aberrometry with a goal of creating the “perfect” wavefront using age-matched norms and algorithms. | This aims to maintain corneal asphericity and minimize laser-induced spherical aberrations. | |
Topography-guided | These procedures are planned around the corneal data and shape. Using a placido disc topographer, this technology measures regular and highly aberrated corneas. | This is the first laser platform dedicated to normalizing the corneal shape while also minimizing refractive error. In addition, these treatments are independent of pupil size and center on the corneal apex rather than the pupil center.1 | |
| |||
LASIK contraindications include central corneal scars, corneal ectasia, ocular surface/corneal infection, recalcitrant dry eye disease (DED), pregnancy and ocular diseases that might limit best-corrected visual acuity.
As technology improves, so does LASIK. A first major improvement was moving from blade flaps (micro-keratome) to laser flaps (femtosecond laser). Laser flaps increase the precision of the flap depth and thickness, leading to improved patient safety. The second big improvement was the development of wavefront-guided and wavefront-optimized treatments and, most recently, topography-guided platforms. These are surgeon-specific technologies, so clinicians should become familiar with the options available at their local surgery centers.
Whether a patient elects to have conventional, wavefront-guided, wavefront-optimized or topography-guided, they can expect great UCVA. Studies comparing LASIK platforms generally show UCVA of 20/20 or better in more than 90% of patients, and the focus shifts instead to low-contrast acuity, measurable higher-order aberrations (HOAs) and other finite differences.5,6
With a well-established track record for safety and visual outcomes, it’s no surprise that many patients present to a new refractive evaluation with a pre-determined mindset that they want LASIK.
Time to SMILE
Despite the slow start for SMILE here in the United States, the newly approved expanded treatment parameters have many thinking now is the time to consider SMILE. The laser creates a lenticule that is extracted through a small opening, effectively flattening the central cornea, similar to an excimer laser ablation in LASIK or photorefractive keratectomy (PRK).
High-energy SMILE (HE-SMILE) was approved in 2016 to treat spherical myopia. This was a great step forward in expanding refractive surgery options, but the energy limitations impacted early visual recovery. Overall, visual recovery was faster than PRK, but slower than LASIK.7 In addition, the initial approval excluded patients with astigmatism.
In March 2018, low-energy SMILE (LE-SMILE), which leads to a LASIK-like quick visual recovery, was approved to treat not only myopia but also myopic astigmatism. The expanded indication includes -1.00D to -10.00D of myopia, up to 3.00D of cylinder and a manifest refraction spherical equivalent up to 11.00D.
One study recently compared both SMILE procedures with LASIK and found that HE-SMILE provided 37% of patients 20/20 vision or better at one day post-op compared with LE-SMILE and LASIK, both of which gave more than 90% of patients 20/20 vision or better in the same timeframe.7 The inclusion of astigmatism treatment and quick visual recovery has led to a rapid increase in SMILE procedures.
Recent peer-reviewed data shows that most LASIK patients experience transient postoperative dryness. The PROWL studies showed LASIK patients were three-times more likely to experience improved, rather than worsening, dryness symptoms.3 Another study also found significantly more LASIK patients had less dryness postoperatively compared with those wearing contact lenses.8
Studies show patients may experience fewer dry eye symptoms with SMILE compared with LASIK because SMILE uses a small opening, not a flap.9-11 Patients may also experience faster recovery of corneal sensation after SMILE compared with LASIK.9-12
Because the procedure preserves more of the anterior stroma, it may also leave the cornea stronger compared with LASIK.11,12 Researchers who looked at the combined effect of corneal hysteresis and corneal resistance factor found SMILE preserved the corneal biomechanical strength better than LASIK.12,13
Interestingly, SMILE allows patients the ability to retreat with LASIK after the initial procedure. For example, a patient can have their small corneal opening turned into a LASIK-like flap.14-16 The other possible option for an enhancement would be PRK.
Currently, more than three million SMILE procedures have been done worldwide.17 Quicker healing times, the micro-invasive corneal opening, greater biomechanical stability and reduced postoperative dryness are why many patients are considering SMILE.3,7-13
 |
|
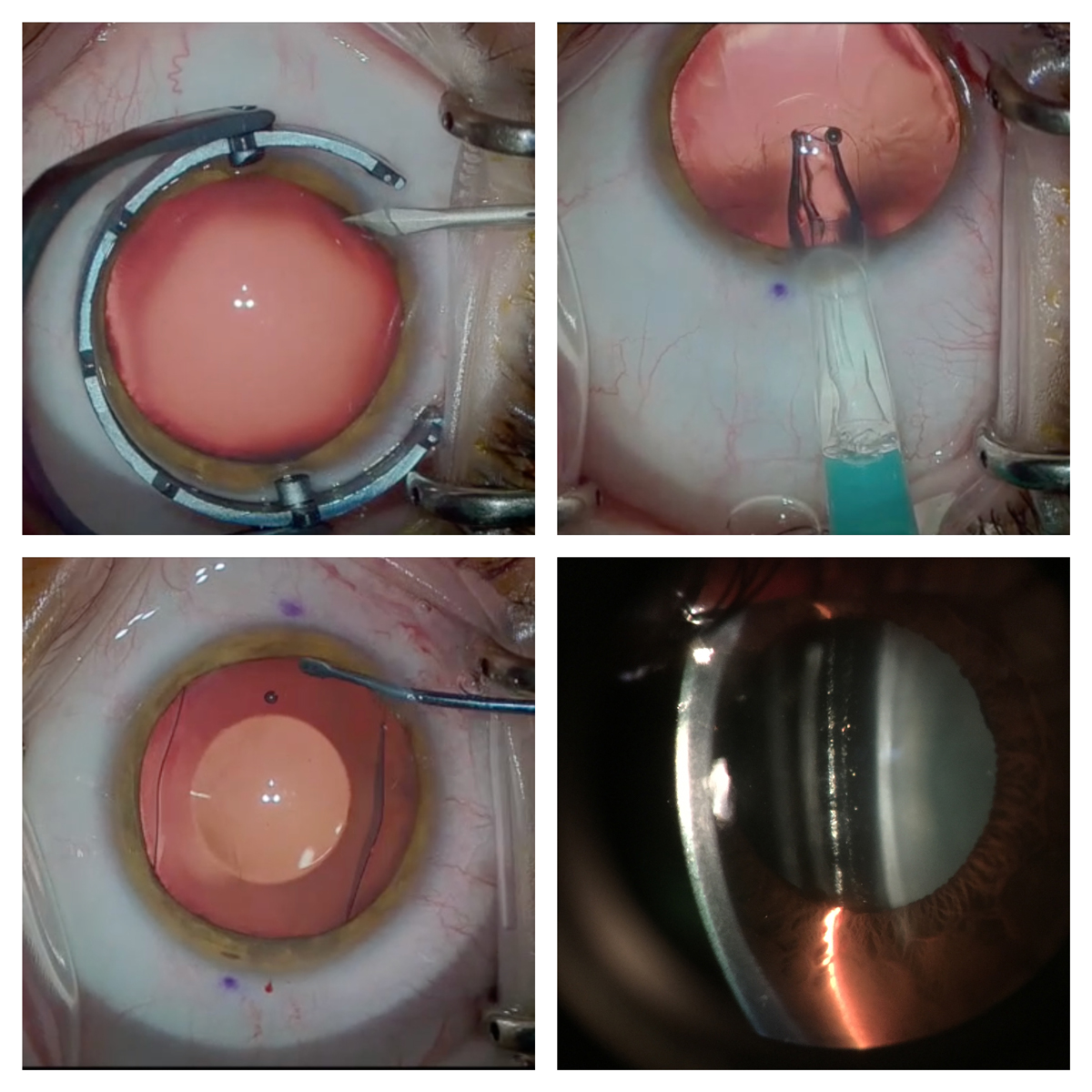
A small opening (top left) is created, and the ICL is inserted into the sulcus (top right) and centered (bottom left). ICL vault over the crystalline lens is evaluated (bottom right). Click image to enlarge. |
A Permanent Contact Lens
The Visian ICL (Staar Surgical) is an additive technology that corrects myopia and myopic astigmatism. It has been available for more than 15 years in the United States, and more than one million patients have opted for this implant. Still, adoption of this technology has been slow.
An ICL can correct myopia between -3.00D and -16.00D and is approved for myopic reduction between -16.00D and -20.00D.18 It can also correct up to 4.00D of cylinder. The procedure is performed by creating a small opening in the cornea, similar to cataract surgery, where the surgeon inserts the soft, folded ICL. The footplates are tucked behind the iris, and the viscoelastic is removed as the ICL sits in the sulcus.
Most eye doctors think of an ICL as an option for patients who don’t qualify for LASIK, such as those with high myopia, thin corneas and irregular topography. ICL surgery is also typically the procedure of choice for patients with DED because of the small opening required.
But the procedure is an important option, even for LASIK candidates. One study comparing wavefront-optimized LASIK with ICLs found that both offered better nighttime contrast sensitivity compared with glasses, but an ICL provided the best nighttime contrast sensitivity.19
These implantable lenses are unique in that they are removable, opening the door for future surgical options as the patient ages. For example, if a -10.00D myope has LASIK, they may not be a candidate for the PanOptix (Alcon) trifocal lens when they need cataract surgery.
Laser refractive surgery for high myopia induces more spherical aberrations and may limit a patient’s candidacy for multifocal intraocular lenses (IOLs). Thus, an ICL is a good option for patients with high myopia because the implant doesn’t change their spherical aberrations or impinge on their candidacy for trifocals later in life.
A patient interested in an ICL must have myopia or myopic astigmatism and an anterior chamber depth above 2.9mm. Preoperatively, these patients require a wet refraction and an orbital ultrasound. When refracting patients with high myopia, use a contact lens for your over-refraction to minimize the impact of vertex factor. Although some doctors size the ICL based on white-to-white measurements, we believe ultrasound allows for more appropriate ICL sizing.
Postoperatively, doctors should carefully check the vault of the ICL (similar to evaluating the vault of a scleral lens with anterior segment optical coherence tomography or a slit lamp) and the patient’s intraocular pressure and ensure the peripheral iridotomies are patent.
New ICL technology remains under investigation in the United States, and the Evo ICL (Staar Surgical) just completed enrollment for its Phase III clinical trial.20 This lens is designed with a central hole to allow aqueous to pass through, eliminating the need for preoperative iridotomies. Outside the United States, the Evo Viva (Staar Surgical), a presbyopic ICL, was just approved as an ICL option for presbyopic myopic patients.21
 |
|
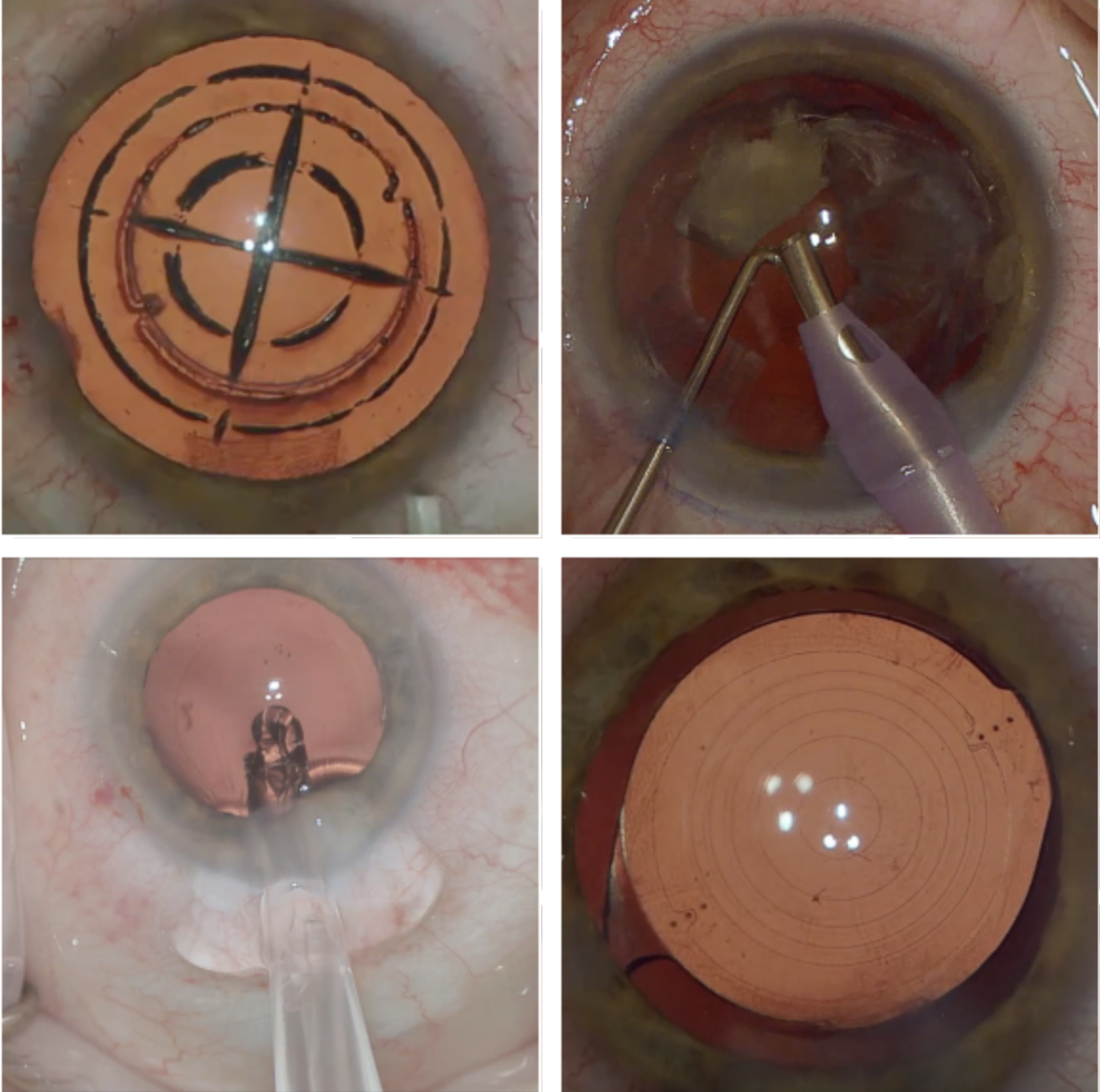
RLE uses a femtosecond laser to soften the lens, create the anterior capsulotomy and create astigmatic keratotomies to correct astigmatism (top left). The cataract or lens is removed by phacoemulsification (top right), and a new lens is inserted (bottom left). Toric IOL placement is evaluated post-op (bottom right). Click image to enlarge. |
Out With the Old
Corneal-based refractive surgery may be an easy decision for many patients, but this isn’t the case for everyone. Often, patients presenting for a refractive surgery evaluation are between the ages of 45 and 55, are at least in the early stages of presbyopia and desire less dependence on glasses at both distance and near. These patients have often wanted refractive surgery for a long time and now have the disposable income to invest. They struggle with bifocal acceptance or have lost more reading glasses than they can count—not an easy clinical picture.
Reshaping the corneal curvature with a laser can change the distance or near power of each eye, but not both. Monovision may be a viable option for these patients, but for candidates who desire excellent depth perception, want to maintain bilateral distance vision or don’t easily accept monovision, their refractive surgery options are limited.
Dysfunctional lens syndrome (DLS)—a newer term that incorporates presbyopia but also focuses on lens HOAs, early light scatter and decreased contrast secondary to lens aging—is a blind spot for laser refractive surgery.22
Staging DLS can be an effective way to stratify patients for laser-based refractive surgery vs. RLE. This option follows the same process and steps as cataract surgery but happens in the absence of visually significant opacification. RLE offers patients with DLS a refractive surgery option to improve distance and near vision while maintaining virgin corneal status. The procedure is also quite safe, with a 6% incidence of adverse events, only 0.9% of which are serious.23
Key candidacy considerations for RLE include patient age, which encompasses the accommodative status of the lens, and preoperative refraction. For myopic patients in general, blended vision or monovision is preferred until a patient reaches their early 50s. In hyperopes, where loss of accommodation is coupled with refractive error, RLE is a viable option for those who are younger than 50.
Although both laser vision correction and RLE can be successful, laser vision correction to steepen a hyperopic eye with early presbyopia tends to offer poorer visual quality and less near range compared with monovision in patients who were previously myopic.
In our practice, we avoid RLE in young myopic patients with a liquified vitreous and in those with a high axial length due to the risk of retinal tears or detachments and peripheral retinal pathology. Conversely, highly hyperopic patients (+4.00D to +6.00D) can do well with RLE, as conventional laser vision correction becomes less predictable.
RLE has three key advantages:
Life-span. Because the cornea is stable in early adulthood, removing the crystalline lens and implanting a fixed-power IOL provides years—if not a lifetime—of refractive stability.
Presbyopic correction. For patients with presbyopia, using a presbyopia-correcting IOL can provide great distance and near vision.
Enhancements. Patients with residual refractive error after a lensectomy and the insertion of a premium IOL can have a cornea-based laser adjustment, when needed, to maximize UCVA.
In our clinic, we have noticed that more patients have chosen RLE this year, perhaps due to the fact that the United States finally has an approved trifocal lens option. The PanOptix now allows properly selected patients to see at distance, intermediate and near without spectacle correction.24 These patients typically have better than 20/25 vision at all distances and, with the same lens in both eyes, they have excellent depth perception.
Embrace the Opportunities
The mask mandate due to the COVID-19 pandemic is driving more patients to seek refractive surgery to escape the problem of spectacle fogging. We should embrace this. Last year, only 20% of all LASIK patients were comanaged by optometrists—that’s approximately $565 million lost in comanagement opportunities with LASIK alone.
In our clinic, we often hear patients say that having refractive surgery was one of the best choices they’ve ever made, right up there with getting married, having kids and buying a house. By keeping up with the latest options and proactively discussing refractive surgery with patients, we can improve their lives and grow our practices.
Dr. Saenz is the clinic and residency director at Parkhurst NuVision in San Antonio, TX, and an adjunct assistant clinical professor at the University of the Incarnate Word’s Rosenberg School of Optometry.
Dr. Ibach is a residency-trained optometrist at Vance Thompson Vision in Sioux Falls, SD. He specializes in anterior segment surgical care, including cataract, corneal disease, glaucoma and refractive surgery.
1. Sandoval HP, Donnenfeld ED, Kohnen T, et al. Modern laser in situ keratomileusis outcomes. J Cataract Refract Surg. 2016;42(8):1224-34. 2. Donnenfeld ED. The best for LASIK. Presented at AAO Subspecialty Day, November 10-11, 2017; New Orleans. 3. Eydelman M, Hilmantel G, Tarver ME, et al. Symptoms and satisfaction of patients in the Patient-Reported Outcomes With Laser In Situ Keratomileusis (PROWL) Studies. JAMA Ophthalmol. 2017;135(1):13-22. 4. Stonecipher K, Parrish J, Stonecipher M. Comparing wavefront-optimized, wavefront-guided and topography-guided laser vision correction. Curr Opin Ophthalmol. 2018;29(4):277-85. 5. Charters L. Wavefront-guided LASIK: bigger increases in corrected/uncorrected VA. Ophthalmology Times. www.ophthalmologytimes.com/view/wavefront-guided-lasik-bigger-increases-correcteduncorrected-va. November 13, 2018. Accessed November 3, 2020. 6. Jain A, Malhotra C, Pasari A, et al. Outcomes of topography-guided versus wavefront-optimized laser in situ keratomileusis for myopia in virgin eyes. J Cataract Refract Surg. 2016;42:1302-11. 7. Nejad MN, Chen AC, Khorrami R, et al. Comparison of early visual outcomes following low-energy SMILE, high-energy SMILE, and LASIK for myopia and myopic astigmatism in the United States. J Cataract Refract Surg. August 25, 2020. [Epub ahead of print]. 8. Price MO, Price DA, Bucci FA Jr., et al. Three-year longitudinal survey comparing visual satisfaction with LASIK and contact lenses. Ophthalmology. 2016;123(8):1659-66. 9. Shen Z, Zhu Y, Song X, et al. Dry eye after small incision lenticule extraction (SMILE) versus femtosecond laser-assisted in situ keratomileusis (FS-LASIK) for myopia: a meta-analysis. PLoS One. 2016;11(12):e0168081. 10. Li M, Zhou Z, Shen Y, et al. Comparison of corneal sensation between small incision lenticule extraction (SMILE) and femtosecond laser-assisted LASIK for myopia. J Refract Surg. 2014;30(2):94-100. 11. Reinstein DZ, Archer TJ, Randleman JB. Mathematical model to compare the relative tensile strength of the cornea after PRK, LASIK, and small incision lenticule extraction. J Refract Surg. 2013;29(7):454-60. 12. Guo H, Hosseini-Moghaddam SM, Hodge W. Corneal biomechanical properties after SMILE versus FLEX, LASIK, LASEK, or PRK: a systematic review and meta-analysis. BMC Ophthalmol. 2019;19(1):167. 13. Zhang Y, Shen Q, Jia Y, et al. Clinical outcomes of SMILE and FS-LASIK used to treat myopia: a meta-analysis. J Refract Surg. 2016;32(4):256-65. 14. Donate D, Thaëron R. Preliminary evidence of successful enhancement after a primary SMILE procedure with the sub-cap-lenticule-extraction technique. J Refract Surg. 2015;31(10):708-10. 15. Liu YC, Rosman M, Mehta JS. Enhancement after small-incision lenticule extraction: incidence, risk factors, and outcomes. Ophthalmology. 2017;124(6):813-21. 16. Chansue E, Tanehsakdi M, Swasdibutra S, et al. Safety and efficacy of VisuMax circle patterns for flap creation and enhancement following small incision lenticule extraction. Eye Vis. 2015;2:21. 17. Zeiss. Zeiss announces 3 million eyes treated with SMILE. eyewire.news/articles/zeiss-announces-3-million-eyes-treated-with-smile. June 9, 2020. Accessed November 3, 2020. 18. US Food and Drug Association. Visian Toric ICL (Implantable Collamer Lens). www.fda.gov/medical-devices/recently-approved-devices/visianr-toric-icl-implantable-collamerr-lens-p030016s001. October 4, 2018. Accessed November 3, 2020. 19. Parkhurst GD. A prospective comparison of phakic collamer lenses and wavefront-optimized laser-assisted in situ keratomileusis for correction of myopia. Clin Ophthalmol. 2016;10:1209-15. 20. ClinicalTrials.gov. Multicenter clinical trial of a phakic implantable collamer lens (ICL). clinicaltrials.gov/ct2/show/NCT04283149. Accessed November 3, 2020. 21. STAAR Surgical introduces EVO Viva presbyopia correcting lens – see young again! Business Wire. www.businesswire.com/news/home/20200707005258/en/STAAR-Surgical-Introduces-EVO-Viva%E2%84%A2-Presbyopia-Correcting-Lens-%E2%80%93-See-Young-Again. July 7, 2020. Accessed November 3, 2020. 22. Durrie D. Dysfunctional lens syndrome, a new way to educate patients. Presented at ASCRS Refract Surg Subspecialty Day, October 14, 2016. 23. Schallhorn JM, Schallhorn SC, Teenan D, et al. Incidence of intraoperative and early postoperative adverse events in a large cohort of consecutive refractive lens exchange procedures. Am J Ophthalmol. 2019;208:406-14. 24. US Food and Drug Administration. AcrySof IQ PanOptix trifocal intraocular lens and AcrySof IQ PanOptix toric trifocal intraocular lens. www.fda.gov/medical-devices/recently-approved-devices/alcon-laboratories-inc-acrysofr-iq-panoptixr-trifocal-intraocular-lens-model-tfnt00-and-acrysofr-iq. Accessed November 3, 2020. |

