 |
It’s becoming clear that glaucoma patients are not compliant with topical medications if there is irritation upon instillation or the integrity of the ocular surface and vision is compromised. Here, I’ll discuss a number of possible solutions to combat these issues.
Medications
Studies have revealed that as many as 59% of patients with glaucoma or ocular hypertension suffer from dry eye disease and that 96% of patients on prostaglandin analog (PGA) medications have meibomian gland dysfunction, as well as a significantly worse ocular surface disease index compared with patients not on PGAs.1,2 Even non-PGA topical medications can disrupt the ocular surface due to preservatives such as benzalkonium chloride (BAK), which is a quaternary ammonium compound that acts as a detergent and can disrupt cell membranes, reduce cell proliferation and decrease corneal epithelial tight junctions.3,4 This detergent disrupts the lipid layer, resulting in a rapid tear film breakup time and the potential for evaporative dry eye.5 Ultimately, patients with ocular surface disease, blurred vision or pain may not remain adherent to the cause—the glaucoma medications.
In addition to its antimicrobial activity, BAK can also exert cytotoxic effects on the ocular surface and several other structures in the eye at concentrations as low as 0.005%.9,10 BAK causes goblet cell loss and negatively affects the survival of cells in the cornea, conjunctiva, retina and the trabecular meshwork and delay corneal wound healing.11-14 BAK is a surfactant and can disrupt the lipid layer of the tear film causing tear osmolarity increase which leads to inflammation.15-17 Chronic exposure to BAK can also result in conjunctival subepithelial inflammation and fibrosis and negatively impact glaucoma surgery outcome.14
Surgeries
Many of the first-line surgeries for primary open- angle glaucoma can be performed by optometrists if they have the credentials, practice and are licensed in any of the 10 states that allow for this. These procedures, including selective laser trabeculoplasty (SLT), can lower intraocular pressure (IOP) by 25% to 30%. SLT is highly effective, relatively noninvasive, quick, easy to perform, repeatable and cost-effective.
Laser peripheral iridotomy is another surgery that can be administered for narrow-angle glaucoma patients.
There is also the bimatroprost sustained-release implant known as Durysta (Allergan), which can often be performed at the slit lamp. To implant the device, prep the ocular surface, place the inserter at the limbus, move toward the center, aiming down, and press the release button. The time-released pellet floats to the lower anterior chamber, where the drug is provided over the next three to four months, although studies show that 28% to 40% of patients have sustained IOP lowering one to two years after implantation.6
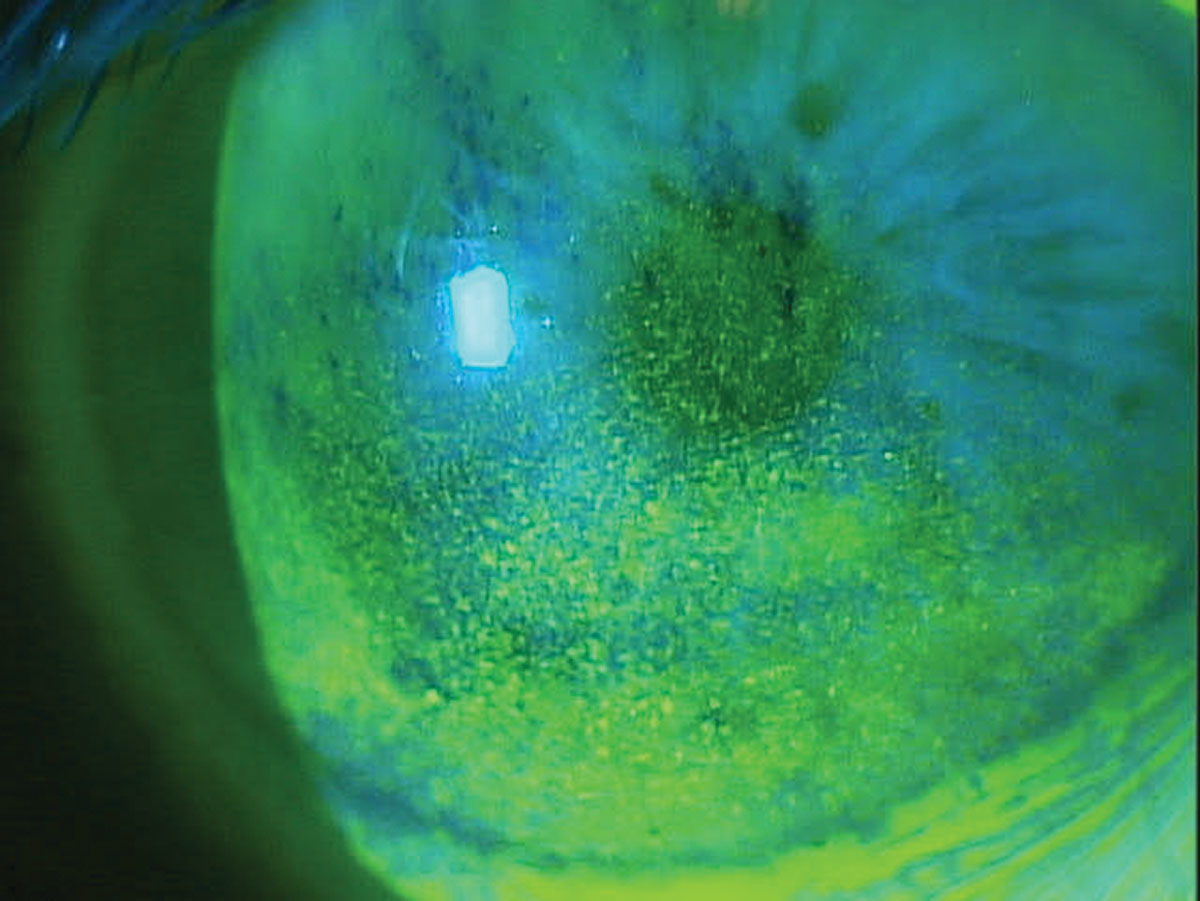 |
|
Corneal superficial punctate keratitis in a patient on multiple topical glaucoma medications. Click image to enlarge. |
MIGS
If your glaucoma patient has significant cataracts, consider minimally invasive glaucoma surgery, or MIGS. Currently, it can only be performed at the time of cataract surgery, so you don’t want to miss the opportunity to lower IOP without drops. Studies have shown as many as 78% of MIGS patients were glaucoma medication-free two years after the procedure, which was 30% more patients than the non-MIGS study group.
Compounded Drops
Using multiple glaucoma drops (including a PGA) with preservatives takes an even greater toll on the ocular surface. An innovative way to resolve this is compounding combination glaucoma drops through ImprimisRx. They are preservative-free and combine various meds in one bottle. This aids the ocular surface by producing less toxicity; it is also simpler for patients to only apply one drop rather than two to four, enhancing patient adherence. Any number of combinations are available depending on what you require for your patient.
Recently, I observed an advanced glaucoma patient I treated with the quad drop QHS (timolol 0.5%, brimonidine 0.15%, dorzolamide 2%, latanoprost 0.005%) and the triple drop in the morning (timolol 0.5%, brimonidine 0.15%, dorzolamide 2%, 10ml bottle). His IOP has remained stable for over three years at 11mm Hg OD and OS, and ocular surface staining has cleared.
PDP-716 (Visiox Pharma, LLC) is the first once-daily brimonidine for the treatment of glaucoma, according to the company, with a PDUFA date of August 4, 2023. The company says due to its once daily dosing regimen, exposure to preservatives for the ocular surface structures will be minimized.
Fewer Drops and Preservatives
To eliminate the harmful effects of BAK and other preservatives, preservative-free formulations were developed. Tafluprost (Zioptan), dorzolamide HCl-timolol maleate ophthalmic solution (Cospt-PF) and Timoptic in Ocudose (Merck) have been available in the US. In December 2022, FDA approved IYUZEH (Théa Pharma, Inc, USA), the first preservative-free latanoprost ophthalmic solution 0.005% for the treatment of primary open-angle glaucoma or ocular hypertension. IYUZEH has been marketed outside of US under the brand name of Monoprost (Laboratoires Théa, France) in 46 countries including Canada.
If one can get fewer drops in the eye, patients would be exposed to less BAK and perhaps experience less toxicity from the PGAs. Our eye only holds about 20µm of fluid and most drop sizes are 30µm to 50µm. One way to reduce the drop volume is through the use of Nanodropper, a novel tip that patients place on their glaucoma medication bottles and reduces the drop size by over 60%. Studies show equal efficacy but less volume means less toxicity and better adherence, not to mention cost savings of extending the bottle life by more than 60%.
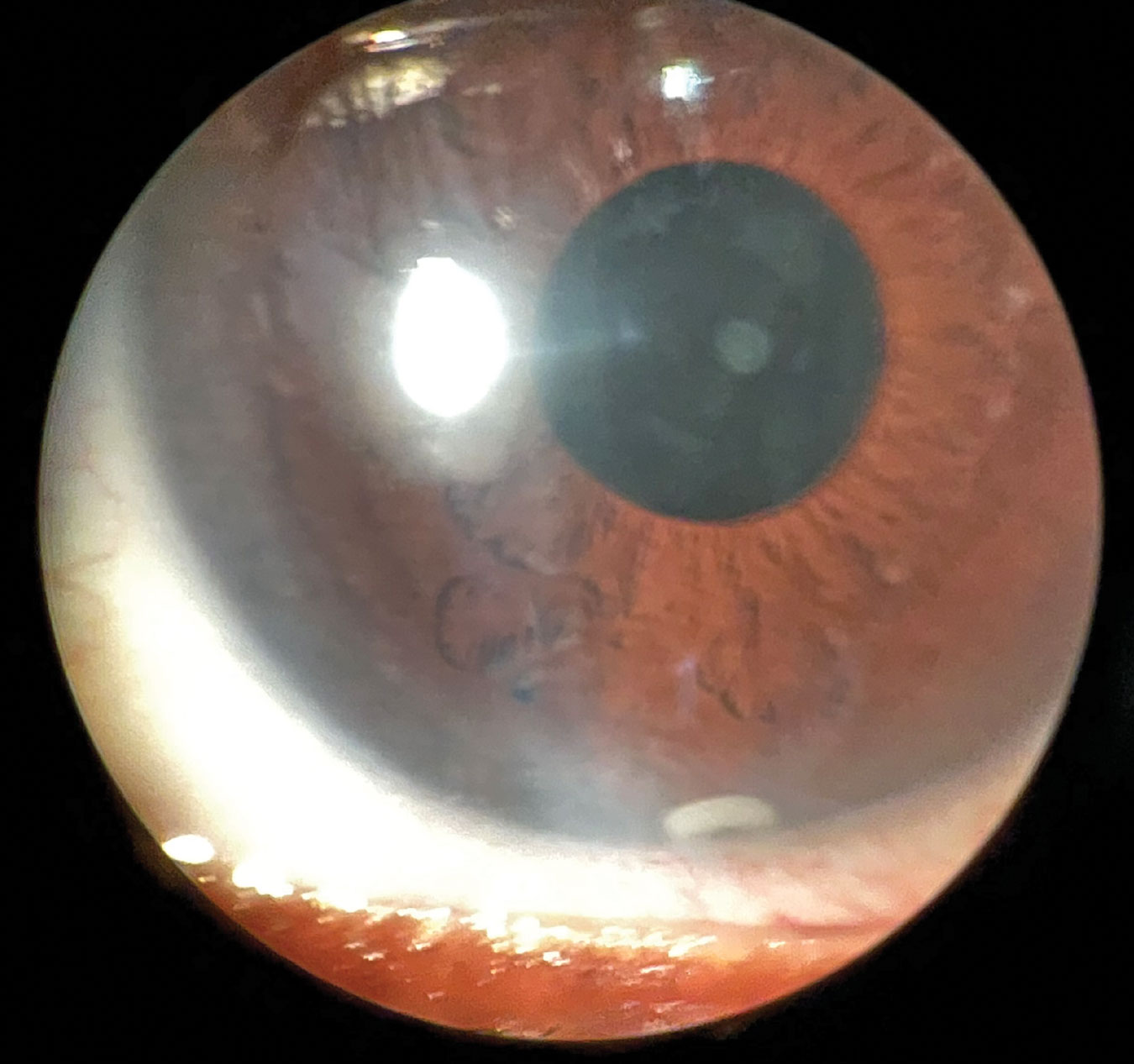 |
|
Anterior segment slit lamp photo of Durysta implant still visible at six-month follow-up. Click image to enlarge. |
The Near Future
TearClear recently announced that it met the primary and all secondary endpoints for its latanoprost 0.005% drop and is on track for a New Drug Application filing with the FDA the first quarter of this year, and numerous other glaucoma drops are to follow. What’s unique about this formulation is that it has BAK in the bottle, but none of it gets to the eye. BAK is a terrific preservative as it maintains sterility, increases stability and aids in solubility—it’s just bad for the ocular surface.
This unique platform allows for the use of a normal bottle (not preservative-free vials or multi-dose bottles that don’t seem to work like normal ones), but the neck of the bottle contains polymers that remove 100% of the BAK as the drop leaves the bottle. So, only the preservative-free medication reaches the eye.
Be Alert
It’s critical for us to observe the ocular surface and meibomian glands closely in all our patients taking chronic glaucoma medications so that we can spot early potential issues involving drug toxicity before adherence is affected. With a plethora of options available, you can help your glaucoma patients maintain a target IOP without compromising their vision, tear film, meibomian glands or ocular surface health.
Dr. Karpecki is the director of Cornea and External Disease for Kentucky Eye Institute, associate professor at KYCO and medical director for Dry Eye Institutes of Kentucky and Indiana. He is the Chief Clinical Editor for Review of Optometry and chairman of the affiliated New Technologies & Treatments conferences. A fixture in optometric clinical education, he provides consulting services to a wide array of ophthalmic clients. Dr. Karpecki’s full disclosure list can be found here.
1. Stewart WC, Stewart JA, Nelson LA. Ocular surface disease in patients with ocular hypertension and glaucoma. Curr Eye Res. 2011;36:391-8. 2. Mocan CM, Uzunosmanoglu E, Kocabeyoglu S, et al. The association of chronic topical prostaglandin analog use with meibomian gland dysfunction. J Glaucoma. 2016;25(9):770-4. 3. Liang H, Pauly A, Riancho L, et al. Toxicological evaluation of preservative-containing and preservative-free topical prostaglandin analogs on a three-dimensional-reconstituted corneal epithelium system. Br J Ophthalmol. 2011;95(6):869-75. 4. Arici MK, Arici DS, Ozec AV, et al. Apoptotic effects of topical antiglaucoma medica-tions on conjunctival epithelium in glaucoma patients. Eur J Ophthalmol. 2014;24(1):63-70. 5. Yee RW. The effect of drop vehicle on the efficacy and side effects of topical glaucoma therapy: a review. Curr Opin Ophthalmol. 2007;18(2):134-9. 6. Craven ER, Walters T, Christie WC, et al.;Bimatoprost SR Study Group 24-month phase I/II clinical trial of bimatoprost sustained-release implant (bimatoprost SR) in glaucoma patients. Drugs. 2020;80(2):167-79. |

