Trouble at the SurfaceMedication, infection, genetics, poor hygiene—these are only a few factors that contribute to ocular surface health. This month in Review of Optometry, experts weigh in on the pathophysiology, diagnosis, treatment and management of numerous diseases affecting this delicate part of the eye. |
For many ocular conditions, history and clinical presentation provide sufficient clues to arrive at an accurate diagnosis and treatment plan with a great degree of certainty. This is not always the case for corneal infiltrative disease, as symptoms and signs can present in similar ways yet beget differing treatment plans.
In order to ensure appropriate outcomes for patients, we have to evaluate all of the clinical clues presented to us, take into account the relevant context, history and symptoms and make an educated decision regarding diagnosis and treatment regimen. A basic understanding of corneal immunity, as well as sterile corneal infiltrative and microbial disease process, is essential for us to make an appropriate diagnosis in a timely fashion and achieve best visual and ocular outcomes for our patients.
Corneal Immune System
The cornea, the eye’s barrier to the outside world, is constantly in contact with microorganisms that may be pathogenic, yet it is able to maintain an excellent degree of resistance to microbial invasion. This resistance consists of both innate and adaptive immunity.
Innate immunity, which begins at the corneal epithelium as the first line of defense, conveys protection within minutes of encounter with an infectious element by releasing cytokines and chemokines. Previously, innate defenses were considered to consist of nonspecific engulfment of pathogens by macrophages, but we now know that innate toll-like receptors confer specificity and enhance discrimination between pathogen and host during the immediate immune response, and keratocytes work with interferons, neutrophils, natural killer cells and macrophages to identify and eliminate the pathogen.1
Adaptive immunity, which may take several days, responds to specific pathogens and antigens by mediating B and T lymphocytes that proliferate and are able to generate memory cells.2 This cellular immune response leads to the migration of white blood cells—including polymorphonuclear leukocytes and mononuclear cells—arising from the limbal arcades and the precorneal tear film into within the cornea, resulting in an infiltrate.3,4
 |
|
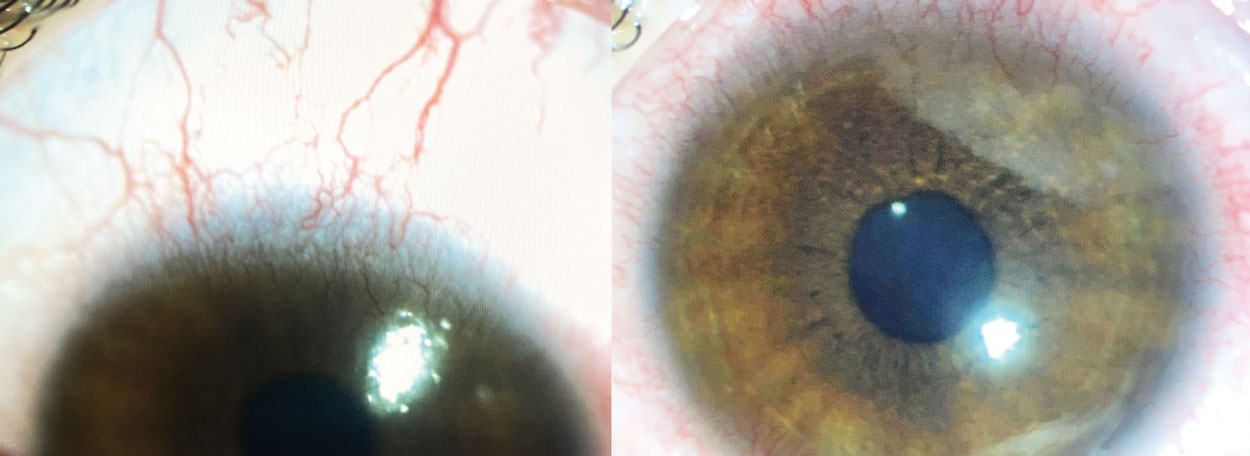
Fig. 1. Contact lens peripheral ulcer (CLPU) in the setting of contact lens over wear. Click image to enlarge. |
Corneal Infiltrates
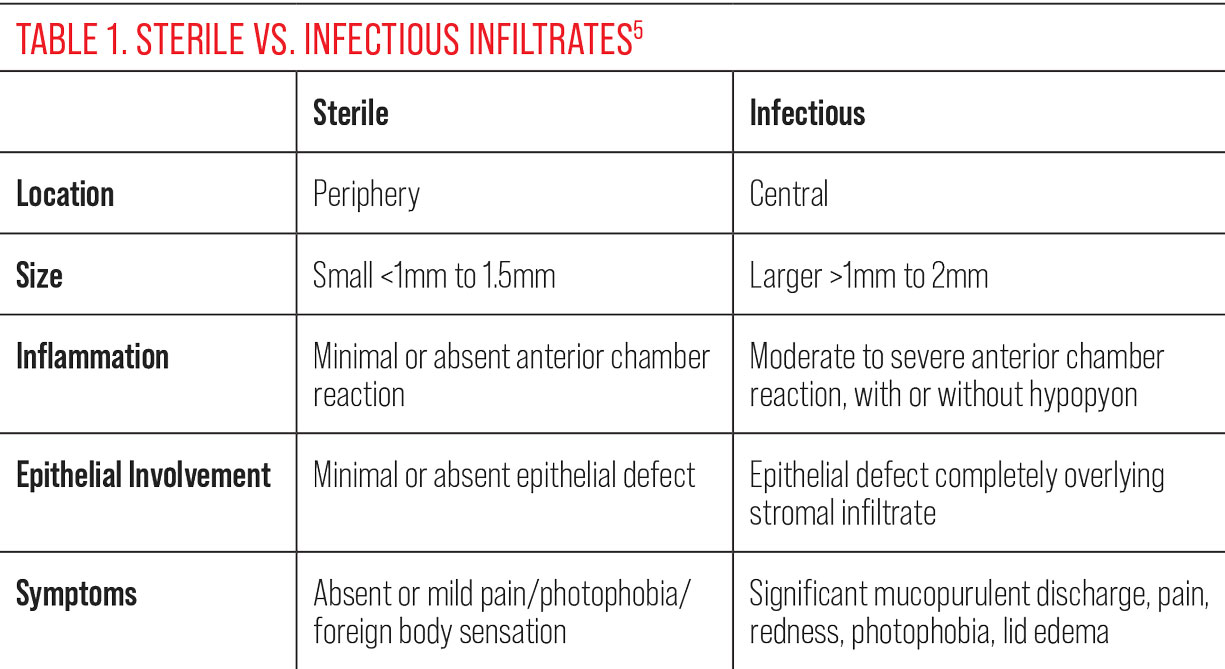
These are often seen with contact lens wear and considered either sterile or infectious, and several distinguishing characteristics need to be evaluated in order to best decide on the etiology (Table 1).1,3 Historically, corneal infiltrative events in the setting of contact lens wear were considered to be sterile in all cases, but we now know that some presumed sterile infiltrates may indeed be culture positive.6
Sterile infiltrates tend to be smaller in size (often less than 1mm to 1.5mm), may be multiple or arcuate, and present with minimal to no pain, epithelial cell staining, conjunctival inflammation, discharge and anterior chamber reaction. These often involve the host or immune response to a microbial antigen in the absence of a live microorganism.6,7 This is in contrast with infected ulcers, which present with increased pain, moderate-to-severe discomfort, discharge, epithelial cell staining, increased size (often greater than 2mm) and moderate or severe anterior chamber reaction.3,5 These are considered to be microbial keratitis, which involves the presence of live proliferating pathogens and the involvement of a secondary immune response.6
Infiltrate size has been found to be less predictive of etiology, as a sizable percentage of culture-positive infiltrates were found to be small in size. Of greater predictive value is the presence of an overlying epithelial defect size, which was found to be present in all cases of culture-positive infiltrates and only about a third of culture-negative cases.5 Noninfectious infiltrates often presented either with no overlying epithelial defect or simply with overlying superficial punctate keratitis.5,8
Most often, sterile infiltrates have been found to be located in the peripheral cornea, often within 4mm of the limbus; they are often subepithelial or anterior stromal in depth.7,8 Sterile infiltrates tend to be nonprogressive and/or self-limiting, responsive to therapy and often do not result in serious sequelae such as an impact on visual acuity, as may be the case with microbial keratitis.3,6-9 In more serious cases, they can result in scarring which can negatively impact visual acuity.3
Sterile corneal infiltrates may be considered marginal, which are self-limiting hypersensitivity reactions due to Staphylococcal antigens in the setting of bacterial eyelid disease; in these cases, the infiltrates are small, circumlimbal, with a 1mm clear interval, often abutting the eyelid margin with mild focal hyperemia.10,11 Marginal infiltrates may be seen in the setting of blepharokeratoconjunctivitis and can result in ulceration of the overlying epithelium.12 Such infiltrates typically respond well to a topical antibiotic-steroid combination drop.
 |
| Click table to enlarge. |
Contact Lens Wear
The infiltrative process may arise from contact lens wear, preservative toxicity, corneal hypoxia, Staphylococcal immune complex formation, poor hygiene and infectious etiologies.8 Risk factors for corneal infiltrative events in the setting of contact lens wear include age under 25 years (with a peak between ages 15 and 25), ametropia, bacterial burden on eyelids, smoking, silicone hydrogel contact lens materials, male gender, refractive error greater than ±5D, limbal redness, previous corneal infiltrative events, overnight and extended soft contact lens wear, use of multi-purpose contact lens solutions and being new to soft contact lens wear.3,6,7,9
In cases of extended contact lens wear, corneal infiltrates often occur in the superior corneal quadrant.7,13 The use of daily disposable contact lenses has been shown to decrease the risk of corneal infiltrative events by over twelvefold in comparison with planned replacement contact lenses.6
Corneal infiltrative events in the setting of contact lens wear can be classified based on their presenting signs and symptoms. According to one study, the most serious and symptomatic category of corneal infiltrative events presents in the form of microbial keratitis.14 This is an infectious corneal event in which there is excavation, diffuse infiltration and necrosis of the epithelium, Bowman’s layer and stroma. Infiltrates are often focal, large and irregular, and may be accompanied by satellite lesions, severe limbal and bulbar redness anterior chamber reaction and mucopurulent discharge. Patients may be rapidly symptomatic for moderate to severe pain, decreased vision, tearing, photophobia and eyelid edema.
Less serious but also clinically significant and symptomatic corneal infiltrative events are the entities known as contact lens–induced red eye (CLARE), contact lens peripheral ulcer (CLPU) and infiltrative keratitis.
• CLARE. Patients are symptomatic for moderate pain, tearing and photophobia on waking. Clinical signs include circumferential hyperemia and multiple focal small infiltrates peripherally without an overlying epithelial defect.
• CLPU. These patients can be asymptomatic, or they can experience moderate pain and/or foreign body sensation. Clinical signs include limbal and bulbar hyperemia, small single focal circular infiltrates less than 2mm in size and in close proximity of the limbus, focal epithelial excavation, infiltration and necrosis of anterior stroma (Figure 1).
• Infiltrative keratitis. Patients are symptomatic for mild to moderate irritation, discharge and redness. Clinical signs include one or multiple small infiltrates within the peripheral anterior stroma, with possible epithelial involvement.
In certain cases, corneal infiltrative events may be asymptomatic and bear little clinical significance. These are usually focal and small, 0.2mm or less, and present with no epithelial staining. Also clinically non-significant and asymptomatic are asymptomatic cases of interstitial keratitis, in which case small focal infiltrates are often seen with epithelial punctate staining and limbal/bulbar redness.6,14
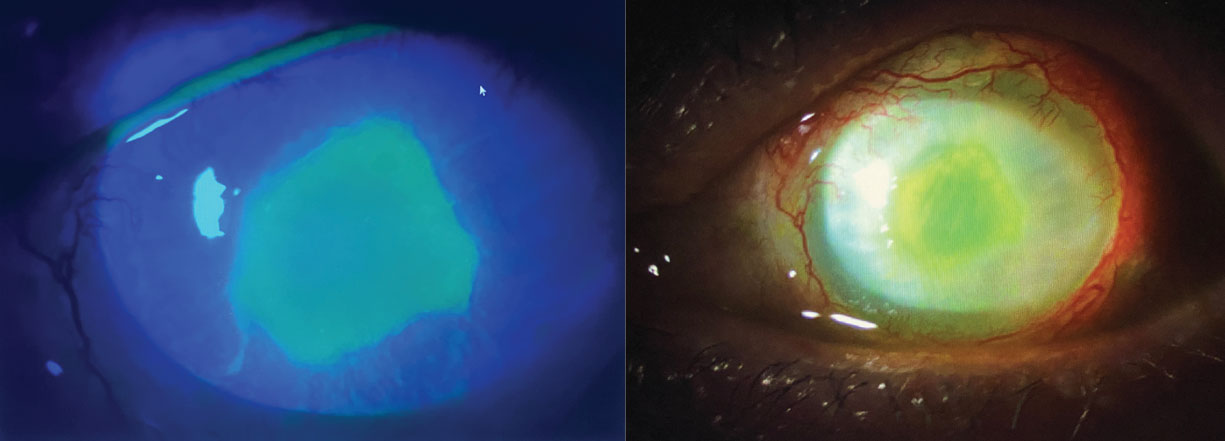 |
Fig. 2. Pseudomonas aeruginosa corneal ulcer. Epithelial defect overlies and mirrors the size of round, central stromal infiltrate. Click image to enlarge. |
Infectious Keratitis
When a stromal infiltrate presents with significant overlying epithelial disruption anterior chamber reaction, along with acute symptoms including pain, redness, discharge, decreased vision and photophobia, particularly in the context of a prior corneal wound disrupting the epithelium, the etiology is more likely to be infectious.15-18 It is important to identify and appropriately treat microbial keratitis, as severe cases may progress to thinning or ulceration of the stroma, corneal perforation, corneal scarring, endopthalmitis and vision loss.16,18,19-21 Infectious keratitis may be due to bacterial, viral, fungal and protozoan etiology.
• Bacterial keratitis. This is often associated with gram-positive organisms including Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pneumoniae and gram-negative organisms including Pseudomonas aeruginosa (Figure 2). The latter is often associated with prolonged daily and extended contact lens wear and can present with a large central epithelial defect overlying a large infiltrate in the setting of severe pain, redness, photophobia, discharge, decreased vision, anterior chamber reaction and hypopyon.5,19,22,23 In cases of bacterial keratitis, an infiltrate is often located centrally or paracentrally and is greater than 1mm with a large and deep epithelial defect extending into the stroma, and may be seen with surrounding corneal edema and multiple satellite lesions.14,24
Factors associated with bacterial keratitis in the setting of contact lens wear include poor contact lens hygiene (including the use of tap water), soft contact lens wear (as there is less tear exchange than with rigid gas permeable contact lens wear and bacterial antigens are allowed to build up), extended contact lens wear and hypoxia secondary to overnight wear.14,24
• Protozoa/parasitic keratitis. In the setting of keratitis, particularly with contact lens wear—while rare—the usual parasitic culprit is Acanthamoeba. This is most often seen in patients who sleep or swim in contact lenses (in pools, hot tubs or freshwater), as well as those that store contact lenses in homemade saline prepared with water.5 Acanthamoeba may have reduced corneal sensation early on, or can first present with severe ocular pain and photophobia; signs may at first be nonspecific, including punctate epithelial erosions, mild inflammation dendritic lesions or a central superficial infiltrate and may progress into a ring infiltrate.25 Acanthamoeba diagnosis can be made with stained smear or culture, as well as by confocal microscopy, PCR or metagenomic sequencing.8, 25
• Fungal keratitis. While rare, fungal keratitis needs to be considered when there is an ulcer in the setting of corneal trauma, particularly with vegetative matter, as well as with contact lens wear or topical corticosteriod use. Fungal ulcers present with deep stromal infiltrates and are often described as having feathery margins and satellite lesions. These ulcers may be accompanied by a hypopyon and need to be considered if there is a prior history of topical steroid use as they can exacerbate the fungal infection.25
• Epidemic keratoconjunctivitis. In the setting of adenoviral epidemic keratoconjunctivitis, multiple small, grayish, nummular, lymphocytic subepithelial infiltrates (SEIs) may develop within the superficial stromal due to an immune reaction (Figure 4). These SEIs generally present bilaterally and in the context of significant hyperemia, eyelid edema, epiphora, chemosis, foreign body sensation, photophobia, membranes, follicular reaction, symblepharon formation and blurry vision.26,27 Systemic signs also include preauricular lymphadenopathy, fever, headache and fatigue.27 Given the effect of SEIs on visual acuity, as well as the potential for vision-threatening scarring, treatment with topical corticosteroids is imperative and is also of significant benefit in the presence of membranes and pseudomembranes. SEI recurrence is possible when steroids are discontinued, so in cases of mild SEI recurrences, topical cyclosporine can be utilized as a steroid-sparing agent.26
• Herpetic. Herpes simplex virus (HSV) and herpes zoster ophthalmicus (HZO) are two viral corneal infections that should be on the list of differentials, particularly if there is a history of herpes zoster or varicella zoster infection, significant recent stress, sun exposure and decreased corneal sensation. HSV keratitis may present with epithelial involvement which can range from nonspecific punctate keratopathy to dendritic ulcers with true terminal end bulbs.28 In cases where HSV involves the stroma, dense stromal edema may be seen with endothelial folds, anterior uveitis and elevated intraocular pressure.28,29 In HZO, epithelial involvement presents with pseudodendrites without true terminal end bulbs.28,29
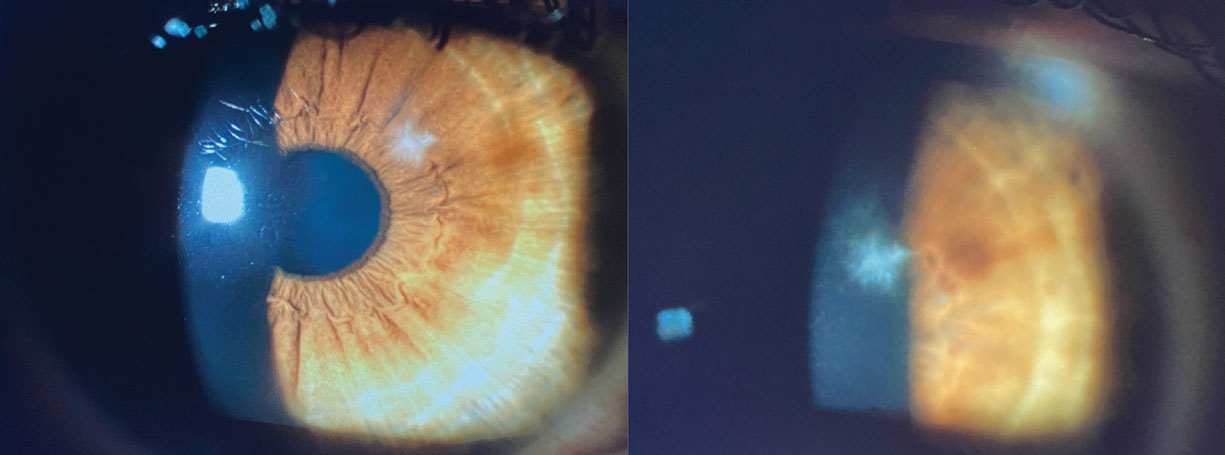 |
Fig. 3. Small, discrete, focal stromal infiltrate masquerading as sterile infiltrate given minimal concomitant signs and symptoms; however, culture positive for yeast. Central location and feathery margins are often seen in fungal ulcers. Click image to enlarge. |
Diagnosis and Treatment
When a patient presents with clinical signs and symptoms of a corneal infiltrate, it is essential to arrive at an accurate diagnosis and initiate appropriate treatment. If the patient wears contact lenses, clinical signs and symptoms on presentation can provide important clues as to the origin of the infiltrate. If the symptoms are mild or absent, the infiltrate is more likely to be sterile. If significant pain, discharge, and/or anterior chamber reaction are present, particularly if there is an overlying epithelial defect, it is reasonable to presume an infectious etiology until proven otherwise and to subsequently obtain scrapings for cultures and smears.5 When infectious etiology is suspected, it is vital to immediately discontinue contact lens wear and to initiate treatment with topical antibiotics with close monitoring and clear return precautions, even if a clear diagnosis has yet to be established, so that treatment is not delayed.5
In the settling of contact lens wear, if the infiltrate is focal and peripheral, less than 1.5mm and without epithelial disturbance, cultures are not required and the infiltrate can be treated with antibiotics or topical antibiotic-steroid combination drops in addition to discontinuation of contact lens wear.7,13 In very mild cases, observation alone may be appropriate. However, if a large overlying epithelial defect is present in the setting of an inflamed eye, a culture is prudent prior to initiating topical antibiotic therapy, followed by steroid treatment 24 to 48 hours after epithelialization has occurred.7
If the patient is developing peripheral corneal infiltrates with extended-wear soft contact lenses, it is essential to refit the patient into daily wear soft or rigid contact lenses.7,8 Rigid gas permeable contact lenses provide superior tear exchange and deposit resistance relative to soft lenses, which is protective against corneal infiltrative events.23,24
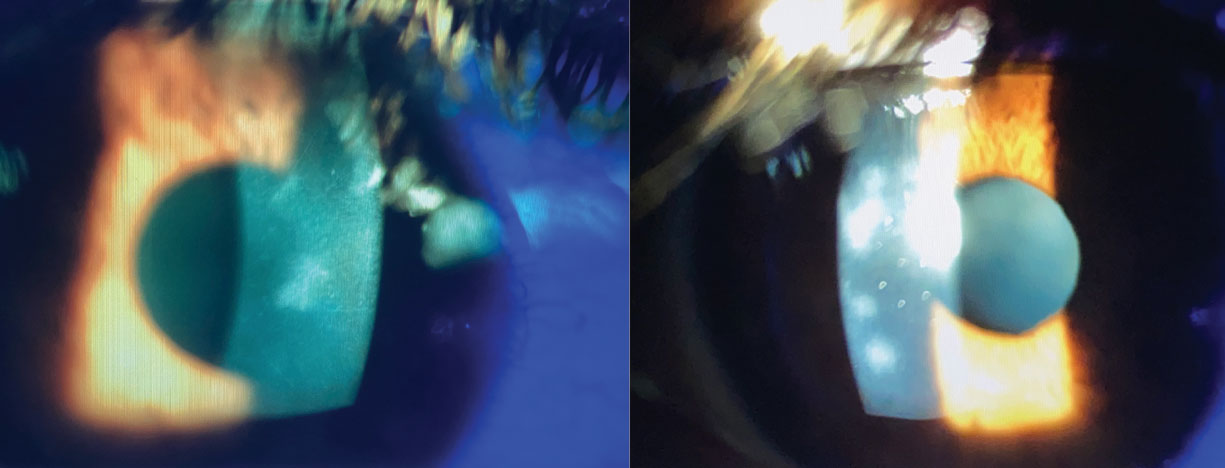 |
|
Fig. 4. Multiple recurrent SEIs following epidemic keratoconjunctivitis. Click image to enlarge. |
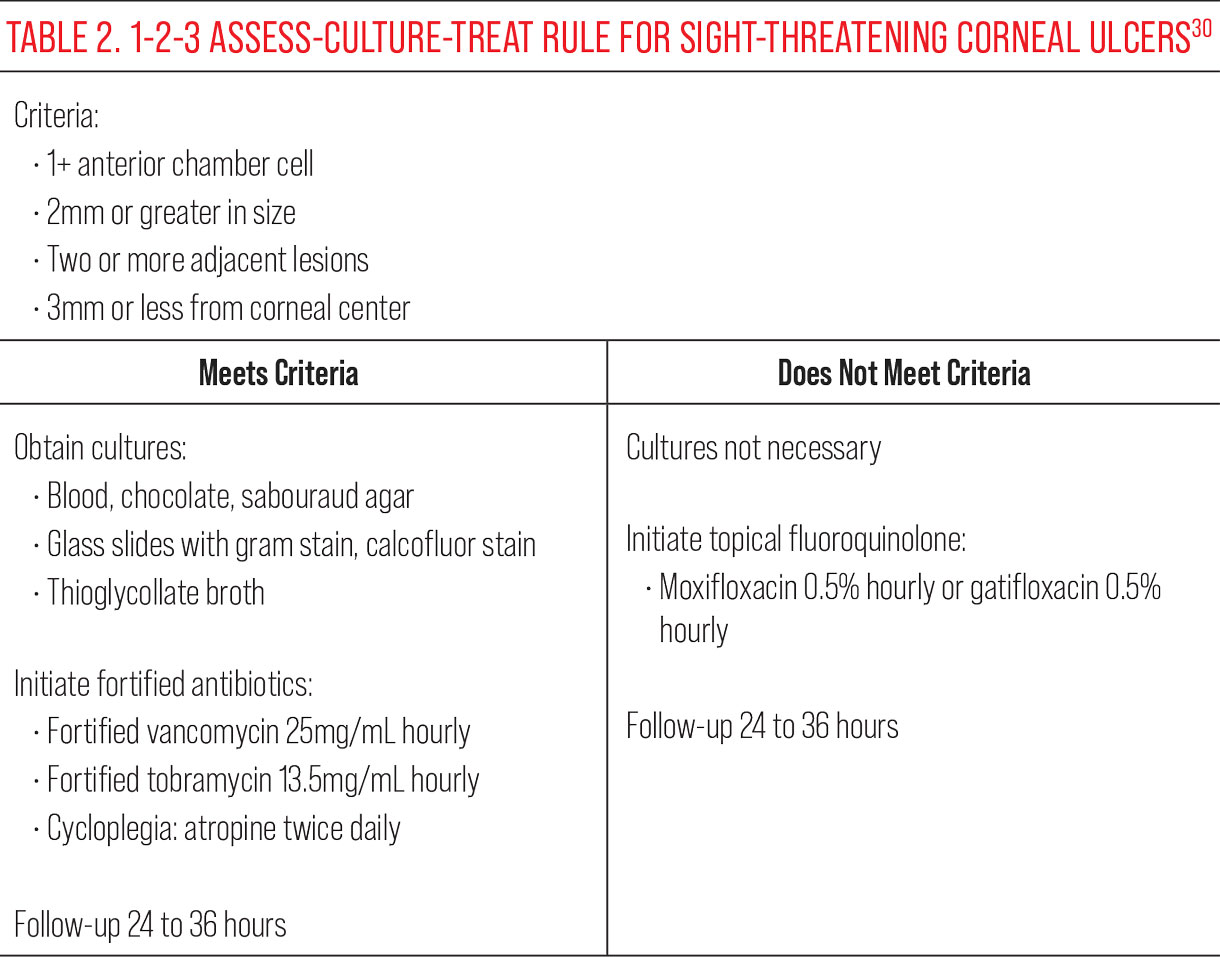
When a patient presents with a corneal ulcer, a systematic algorithm for assessment and treatment—called the 1-2-3-ACT (Assess, Culture, Treat) Rule, originally adapted by the Massachusetts Eye and Ear Infirmary—can be used to identify sight-threatening ulcers and manage them accordingly (Table 2).
A corneal ulcer that meets criteria for the 1-2-3 rule will present with any one of the following criteria: 1+ or greater anterior chamber cell, 2mm or greater, two or more adjacent lesions, and/or 3mm or less distance from the corneal center. If the ulcer meets this criteria, it is recommended to obtain cultures (blood, chocolate and sabouraud agar, gram stain and calcofluor stains on glass slides, and thioglycollate broth), followed by initiation of fortified antibiotics (vancomycin 25mg/mL and tobramycin 13.5mg/mL) hourly in the affected eye in addition to twice daily topical cycloplegia with atropine.30 Follow-up should be arranged within 24 to 36 hours with a provider trained in corneal pathology. Additional indications for corneal culturing include a recent history or ocular trauma, topical or systemic corticosteroid use or immunocompromised status.
For corneal ulcers that do not satisfy the criteria of the 1-2-3 rule, it is reasonable to presume the ulcer is not immediately sight-threatening, forgo culture and begin empirical treatment with a topical fluoroquinolone, such as moxifloxacin 0.5% or gatifloxacin 0.5%, one drop hourly. Follow-up should occur within 24 to 36 hours to monitor progress.30
 |
|
Click table to enlarge. |
Takeaways
When patients with presumed corneal infiltrative and infectious disease present for evaluation, a lengthy list of differential diagnoses can come to mind. In order to narrow down the correct differential and provide appropriate treatment, it is essential to carefully consider patient history, symptoms and clinical corneal signs in the context of other ocular findings. If the diagnosis is unclear but clinical signs and symptoms are significant, it may be prudent to first obtain confirmatory testing, such as cultures and smears, and then immediately initiate empirical treatment for an infectious etiology until a definitive answer arrives. Comanagement with corneal ophthalmology subspecialists may be essential, particularly if the signs are worsening or a visually threatening event is imminent.
Dr. Cherny is an instructor in ophthalmology at the New York Eye and Ear Infirmary of Mount Sinai. She specializes in complex contact lens fittings, anterior segment and corneal surgery comanagement, as well as primary and emergent eye care. Dr. Cherny is a fellow of the American Academy of Optometry. She has no financial disclosures.
1. Akpek EK, Gottsch JD. Immune defense at the ocular surface. Eye. 2003;17:949-56. 2. Kumar A, Yu FS. Toll-like receptors and corneal innate immunity. Curr Mol Med. 2006;6(3):327-37. 3. Jansen ME, Situ P, Begley CG, et al. Characterizing contact lens-related corneal infiltrates: a pilot study. Cornea. 2016;35(12):1578-83. 4. Bazan NG, Bazan HP. Ocular responses to inflammation and the triggering of wound healing: lipid mediators, proto-oncogenes, gene expression and neuromodulation. In: Bazan NG, ed. Lipid Mediators in Eye Inflammation. Braquet P, ed. New Trends in Lipid Mediators Research, 5th ed. Basel: Karger; 1990:168-80. 5. Stein RM, Clinch TE, Cohen EJ, et al. Infected vs. sterile corneal infiltrates in contact lens wearers. Am J Ophthalmol. 1988 ;105(6):632-6. 6. Steele KR, Szczotka-Flynn L. Epidemiology of contact lens-induced infiltrates: an updated review. Clin Exp Optom. 2017;100(5):473-81. 7. Donshik PC, Suchecki JK, Ehlers WH. Peripheral corneal infiltrates associated with contact lens wear. Trans Am Ophthalmol Soc. 1995;93:49-60; discussion 60-4. 8. Suchecki, JK, Ehlers WH, Donshik, PC. Peripheral corneal infiltrates associated with contact lens wear. CLAO Journal. 1996;22(1):41-6. 9. Chalmers RL, Wagner H, Mitchell GL, et al. Age and other risk factors for corneal infiltrative and inflammatory events in young soft contact lens wearers from the Contact Lens Assessment in Youth (CLAY) study. Invest Ophthalmol Vis Sci. 2011;52(9):6690-6. 10. Mondino BJ. Inflammatory disease of the peripheral cornea. Ophthalmology. 1988;95(4):463-72. 11.The American Academy of Ophthalmology. Immune-related disorders of the external eye. In: External Disease and Cornea (Basic and Clinical Science Course). American Academy of Ophthalmology; 2016-2017:195. 12. Mack HG, Fazal A, Watson S. Corneal ulcers in general practice. Aust J Gen Pract. 2022;51(11):855-60. 13. Baum J, Dabezies OH. Pathogenesis and treatment of “sterile” midperipheral corneal infiltrates associated with soft contact lens use. Cornea. 2000;19(6):777-81. 14. Sweeney DF, Jalbert I, Covey M, et al. Clinical characterization of corneal infiltrative events observed with soft contact lens wear. Cornea. 2003;22(5):435-42. 15. Ni N, Srinivasan M, McLeod SD, et al. Use of adjunctive topical corticosteroids in bacterial keratitis. Curr Opin Ophthalmol. 2016;27(4):353-7. 16. Palioura S, Henry CR, Amescua G, Alfonso EC. Role of steroids in the treatment of bacterial keratitis. Clin Ophthalmol. 2016;10:179-86. 17. Moshirfar M, Hopping GC, Vaidyanathan U, et al. Biological staining and culturing in infectious keratitis: controversy in clinical utility. Med Hypothesis Discov Innov Ophthalmol. 2019;8(3):145-51. 18. Lin A, Rhee MK, Akpek EK, et al. Bacterial keratitis preferred practice pattern. Ophthalmology. 2019;126(1):P1-55. 19. Fortingo N, Melnyk S, Sutton SH, et al. Innate immune system activation, inflammation and corneal wound healing. Int J Mol Sci. 2022;23(23):14933. 20. McDonald EM, Ram FS, Patel DV, McGhee CN. Topical antibiotics for the management of bacterial keratitis: an evidence-based review of high quality randomized controlled trials. Br J Ophthalmol. 2014;98(11):1470-7. 21. Austin A, Lietman T, Rose-Nussbaumer J. Update on the management of infectious keratitis. Ophthalmology. 2017;124(11):1678-89. 22. Cohen EJ. Management of small corneal infiltrates in contact lens wearers. Arch Opthalmol. 2000;118(2):276-7. 23. Bourcier T, Thomas F, Borderie V, et al. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Brit J Ophthalmol. 2003;87(7):834-8. 24. Carnt N, Samarawickrama C, White A, Stapleton F. The diagnosis and management of contact lens-related microbial keratitis. Clin Exp Optom. 2017;100(5):482-93. 25. The American Academy of Ophthalmology. Infectious diseases of the external eye: microbial and parasitic infections. In: External Disease and Cornea (Basic and Clinical Science Course). American Academy of Ophthalmology; 2016-2017:152-7. 26. Arici C, Sultan P, Mergen B. Evaluation of the impact of subepithelial corneal infiltrates on corneal biomechanics after epidemic keratoconjunctivitis. Arq Bras Oftalmol. 2022;85(5):478-84. 27. Bawazeer, Ahmed. Epidemic keratoconjunctivitis. Medscape. http://emedicine.medscape.com/article/1192751-overview#a0101. March 20, 2013. Accessed September 28, 2023. 28. Krachmer JH, Mannis MJ, Holland EJ. Cornea. St, Louis, MO: Mosby/Elsevier; 2011. 29. Rapuano C. Corneal infections, inflammations and surface disorders. In: Color Atlas and Synopsis of Clinical Ophthalmology. Wills Eye Institute. Philadelphia: Wolters Kluwer; 2012:188. 30. Ung L, Wang Y, Vangel M, et al. Validation of a comprehensive clinical algorithm for the assessment and treatment of microbial keratitis. Am J Ophthalmol. 2020;214:97-109. |

