As we watch the ramp-up of COVID-19 vaccination, we can marvel at the ingenuity that brought these vital agents to market in under a year. But it also starkly underlines the importance of vaccines as a whole to society. Unfortunately, a decline in routine vaccination was among the unwelcome consequences of the pandemic lockdowns that began in March 2020, which significantly altered health care as we know it. Patient visits for routine and emergency care began to decrease.1 This has resulted in a reduced rate of vaccinations for vaccine-preventable diseases (VPD) across the nation.
For individuals 18 years and younger, vaccine rates have been down by as much as 30% compared to previous years, although the rate for newborns remained generally stable (with some variability for individual states).2 On a global scale, the disruption to vaccine distribution programs and access has also been altered.3 The World Health Organization estimates that globally, 94 million children will not receive the measles vaccine because of COVID-related closures and decreased access to care. A recent report stated that measles cases in 2019 were the highest they have been since 1996, and are expected to rise.3
 |
|
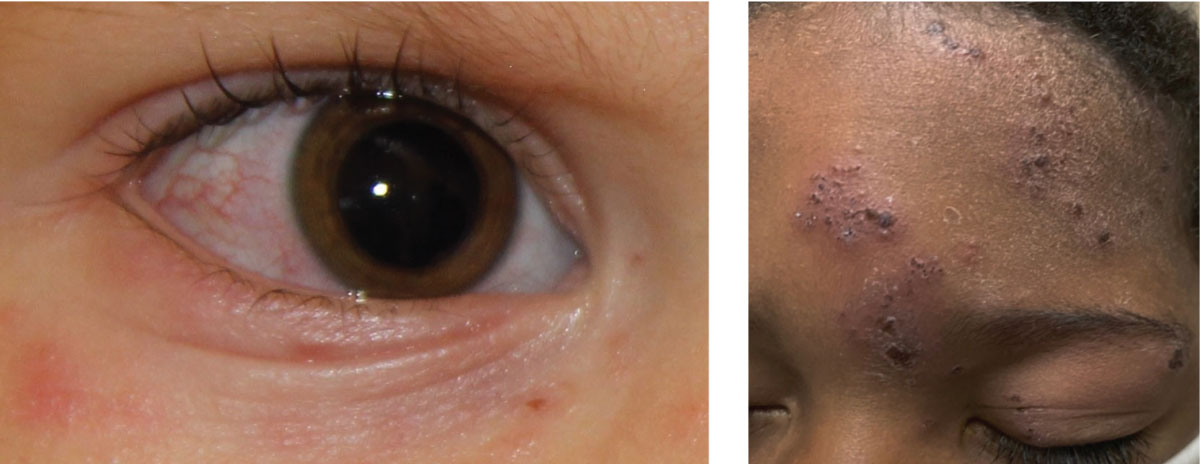
Fig. 1. At left, ocular involvement of herpes zoster with associated rash of the ocular adnexa and surrounding skin; above, classic presentation of herpes zoster. Click image to enlarge. |
With the decrease in vaccine rates, both in the US and globally, there is increased potential for reemergence of VPD, particularly in children. VPDs have significant and wide-ranging systemic effects (including ocular manifestations) with the potential to be fatal, so the decrease in vaccine rates globally has significant ramifications for both at-risk individuals and health care providers.
This article provides an overview of the ocular manifestations of many VPDs so that we can be vigilant for them in appropriate patients.
Ocular Signs of VPD
Pediatric vaccine-preventable diseases may present with ocular involvement, but more often will present with systemic signs or symptoms. Symptomatic patients usually present to the pediatrician, primary care practitioner or emergency medicine provider. Eye care providers (ECPs) in hospital or multidisciplinary settings may be more likely to encounter these cases as part of the patient care team, but sequelae of primary infections may present to ECPs in all settings, including community-based optometrists.
The Value of VaccinesThe goal of any vaccine is twofold: first, to keep the individual healthy and prevent future disease, and second, to halt spread within a population by increasing herd immunity. This is especially necessary for protecting vulnerable populations such as infants, pregnant women and immunocompromised individuals. The amount of herd immunity needed to stop the spread of infection varies by the contagiousness of the disease. This is defined by the basic reproduction number (R0), which is the average number of individuals a single person is likely to infect in a completely susceptible population.4 The higher the R0 value, the more contagious the disease—and therefore the increased importance of vaccinations. The Centers for Disease Control and Prevention (CDC) has a recommended vaccine schedule for people of all ages. The highest number and frequency of vaccinations are recommended during childhood. Most vaccines require several doses throughout the first six years of life (e.g., DTap, MMR, polio, hepatitis A and B, pneumococcal, varicella, meningococcal), some require boosters and some—namely, influenza—require an annual administration. |
While the following conditions are rare in optometric practice, they might be considered in abnormal red eye presentations, or in cases where acute disease does not resolve with the typical treatment regimen. Providers may elect to add questions about vaccine history to intake questionnaires and consider VPDs when other systemic conditions present concurrently, especially rashes or persistent coughing.
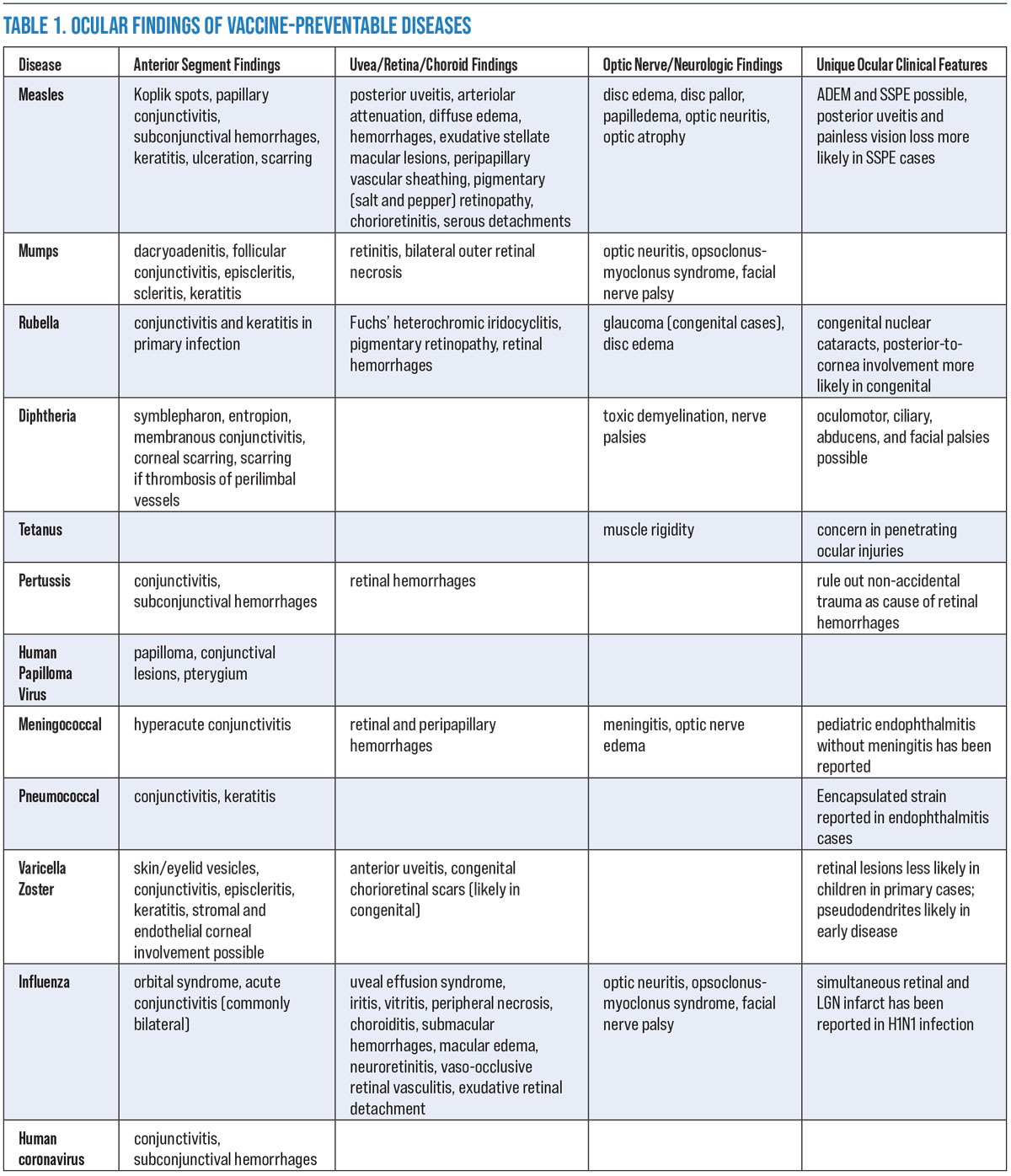 |
| Table 1. Ocular findings of vaccine-preventable diseases. Click image to enlarge. |
Measles
This viral infection is highly transmissible due to its high R0 value and the trend for declining vaccine rates. There were several recorded outbreaks in the United States prior to the COVID-19 pandemic.5 Measles infection has an incubation period of nine to 10 days followed by systemic signs, which include high fever, cough and Koplik spots (blue-gray lesions on an erythematous base) with an eventual onset of the classic skin rash.
Ocular manifestations include a non-purulent papillary conjunctivitis. Rarely, Koplik spots can be visualized on the conjunctiva. Keratitis leading to scarring and blindness can occur and is more likely in patients with poor vitamin A intake. Other reported ocular manifestations include subconjunctival hemorrhages, posterior uveitis and pigmentary retinopathy.5-9 Measles-related posterior uveitis is characterized by disc swelling, arteriolar attenuation, diffuse retinal edema and scattered retinal hemorrhages with exudative stellate macular lesions.
 |
|
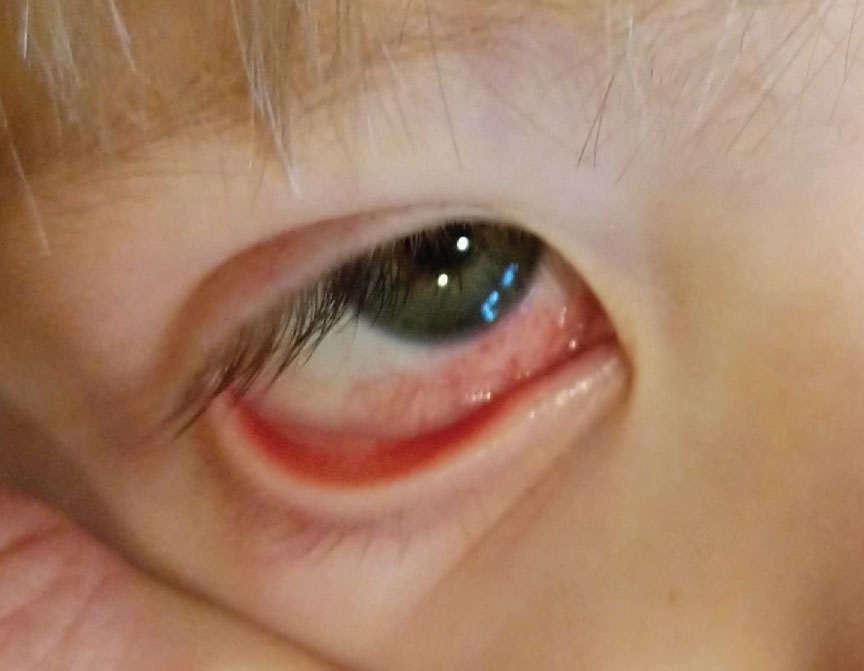
Fig. 2. Sectored red-eye presentation in a three-year-old. All pediatric red eyes, especially unusual presentations, should have a full vaccine history included in the work-up. Click image to enlarge. |
Measles retinopathy is usually bilateral and occurs in immunocompetent patients. Upon resolution, signs such as disc pallor, peripapillary vascular sheathing and pigmentary changes result in a “salt and pepper” fundus appearance. In congenital cases, the electroretinogram (ERG) may be normal. In acquired cases, the ERG is reduced during active infection and can improve with time. Neuroimaging may show white matter abnormalities. Secondary outcomes of viral infection, such as acute disseminated encephalomyelitis (ADEM), have also been reported.5-9
A rare sequela of a mutant variant of measles relevant to all ECPs is subacute sclerosing panencephalitis (SSPE.) Patients are school-aged children whose primary infection occurred before age two. While the condition is rare, early SSPE mimics typical pediatric malingerers. Signs include painless vision loss and possibly chorioretinitis starting in the macula or posterior uveitis. Ocular findings are common and occur in up to 50% of cases.
SSPE results in cognitive decline and eventually death. Visual symptoms and retinal findings precede neurologic findings by weeks to years. Associated findings of focal necrotizing retinitis, ground-glass retinal whitening, RPE mottling, papilledema, optic atrophy, retinal folds, retinal hemorrhages, serous detachments and occlusive vasculitis with minimal vitreous inflammation have all be described.5-9
Mumps (Parotiditis)
Though primary infections of mumps are less common in the United States, sporadic outbreaks have been reported. The severity of the systemic illness is variable; prior to vaccine development 15% to 27% of infections were asymptomatic. After an incubation period of 12 to 25 days, patients develop parotiditis, fever, headache, muscle aches and malaise. Reported ocular signs include dacryoadenitis, follicular conjunctivitis, episcleritis, scleritis, keratitis, retinitis, optic neuritis, opsoclonus-myoclonus syndrome and facial nerve palsy.5,10-20
Rubella
This often presents to the eye care practitioner as a sequelae of a primary or congenital exposure. In primary cases, ocular signs are mild. Conjunctivitis is common, though central epithelial keratitis has also been reported. Fuchs’ heterochromic iridocyclitis—a unilateral uveitis with a triad of heterochromia, predisposition to cataract and glaucoma, and keratic precipitates—is associated with rubella infection. In congenital cases, severity is inversely related to gestational age at the time of maternal infection.
The triad of congenital rubella syndrome includes auditory, cardiac and ocular defects, which occur in 30% to 40% of cases. Nuclear cataracts, microphthalmos and congenital glaucoma are more likely in the first trimester. The most common outcome is pigmentary retinopathy, which can occur when maternal exposure occurs before gestational age of 20 weeks. Rubella retinopathy is usually benign, non-progressive and visually insignificant, though rare complications such as hemorrhages and disc edema can reduce vision.
Diphtheria
Once a leading cause of childhood death, widespread vaccine use has led to near eradication of diphtheria in the United States. As such, reports in the literature of ocular involvement are sparse but generally address two areas of concern: membranous conjunctivitis and nerve dysfunction secondary to toxic demyelination. Diphtheria conjunctivitis is described in the literature as both pseudomembranous and membranous, resulting from formation of a gray plaque of necrotic tissue. Though involvement of extra-respiratory sites is uncommon, this severe conjunctivitis can cause secondary problems such as symblepharon, conjunctival and corneal scarring, and other ocular surface irregularities.
Tetanus
This anaerobic infection is generally a concern in emergency cases of penetrating ocular injuries. Clostridium tetani spores can germinate, leading to production of toxins that act on the nervous system, resulting in the well-known associated muscle rigidity.5,26,27
Pertussis
Also known as whooping cough, this condition generally affects the eye in one of two ways. The most common ocular sign is a subconjunctival hemorrhage resulting from the characteristic cough. Less commonly, retinal hemorrhages have been reported, though it is essential in all pediatric cases involving retinal hemorrhages to fastidiously rule out non-accidental trauma. Although not currently employed as such, the conjunctiva has been proposed as a site for vaccine delivery.5,26,27,30,31
Human Papilloma Virus (HPV)
Vertical and contact transmissions of HPV are the most likely routes of ocular infections in children, though several routes exist, including sexual contact and autoinoculation. HPV is categorized into high risk and low risk subtypes; the former leads to anogenital and cervical cancers while the latter causes dysplasia that does not progress to cancer. HPV has been found in conjunctival and eyelid lesions such as inverted papillomas and pterygia. While both subtypes have been identified in ocular tissue, low-risk HPV is more commonly associated with ocular lesions.5,6,33-39
Meningococcus
Meningococcal disease earns its name due to its strong association with meningitis. While it is not the only organism which causes meningitis, Neisseria meningitidis is a leading cause of bacterial meningitis in babies, children and adults.
Pediatric cases of endophthalmitis without meningitis have also been reported. Other reported ocular signs include severe photophobia, hyperacute conjunctivitis, retinal and peripapillary hemorrhages, and optic nerve edema. Systemic signs can include fever, headache, neck stiffness, vomiting, lethargy and seizure. Meningococcal disease is potentially blinding and fatal and must be quickly treated both reactively and proactively. In these cases, immediate referral to emergency care is warranted.5,40-46
Pneumococcus
In children, pneumococci are generally involved in cases of bacterial meningitis and acute otitis media, but cases of pneumococcal conjunctivitis, keratitis and endophthalmitis have been reported. Pneumococcus is found in one third of bacterial keratitis cases.5,47-56
Varicella Zoster Virus (VZV)
Primary varicella infections have dropped dramatically since 1995, when the VZV vaccine was introduced, though reactivation of infections such as herpes zoster ophthalmicus (HZO) can occur. Postherpetic neuralgia is less likely in children than adults.
Primary VZV infections can cause eyelid lesions (Figure 1), conjunctivitis, episcleritis, keratitis and anterior uveitis. Retinitis, common in adults, is rare in children in primary infection, though congenital VZV infection can leave discrete white chorioretinal scars. After vaccination, mild non-infectious keratitis has been reported.
Those who have had a primary VZV infection and those who received the vaccine are less likely to develop HZO. As is the case with adults, early anti-viral treatment, close follow-up and careful corneal management is important in pediatrics. Children who are immunocompromised or have systemic disease should be hospitalized and treated with IV antiviral therapy. Damage to ocular structures in the course of pediatric HZO can lead to deprivational amblyopia.5,6,57-58
 |
|
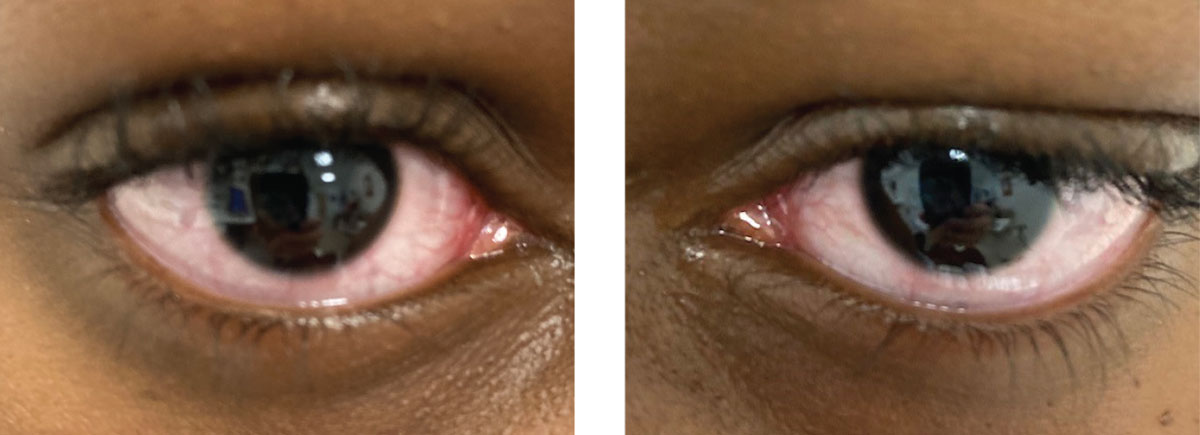
Fig. 3. Same patient, right and left eyes, respectively. Typical presentation of acute-onset red eyes in a school-aged child. Click image to enlarge. |
Influenza
The seasonal flu can produce the following ocular signs: orbital inflammatory syndrome, acute conjunctivitis, conjunctival injection, anterior chamber inflammation, uveal effusion syndrome, vitritis, vasculitis, frosted branch angiitis, macular edema, submacular hemorrhaging, exudative retinal detachment, retinopathy, peripheral retinal necrosis, choroiditis, neuroretinitis and optic neuritis. Pain and decreased vision have been reported. Visual acuity loss has also been reported as the presenting sign of simultaneous retinal and lateral geniculate body infarct.
Influenza is unique in that a yearly vaccine is needed to reduce contraction, as the virus itself mutates frequently. However, both severity and duration of symptoms tend to be reduced in individuals who have received the vaccine.5,59,60
Human Coronavirus
Though they only became a household name in 2020, human coronaviruses are quite common, and often lead to fever, fatigue, rhinitis, headache, sore throat and other common cold-type symptoms. The hallmark signs of COVID-19 additionally include shortness of breath, and loss of taste or smell.
While literature on SARS-CoV-2 continues to develop, there are several reports of COVID-19 conjunctivitis developing in individuals who test positive for SARS-CoV-2. Red eyes generally present as a typical viral conjunctivitis, with a unilateral presentation that transitions to bilateral, watery discharge, and mild to moderate redness.61,62 Children, especially those under 10 years of age, continue to have lower reported symptomology from COVID-19, although they may be asymptomatic carriers. Those who do show symptoms can rarely develop multi-system inflammatory syndrome in children (MIS-C).
Most reports of this condition show high levels of antibodies to SARS-CoV-2, indicating a previous infection. Associated signs and symptoms include fever in 100% of cases and bilateral conjunctival injection in 55% of cases, in addition to significant gastrointestinal, cardiovascular, hematologic and respiratory involvement; however, there is a high rate of recovery for these patients.63
There are several theories as to why children are less impacted by the coronavirus. One suggestion relates to the spike glycoprotein, which is present on the envelope of the coronavirus and helps the virus bind to ACE2 receptors. It has been theorized that children perhaps have less (or immature) ACE2 receptors, which reduces binding of the virus.64 Another theory is that the proteins on the spike glycoprotein are similar to those found in measles and rubella. Since children are frequently exposed to both coronaviruses and the measles/rubella proteins through routine vaccinations, their immune systems may be able to ward off the Sars-CoV-2 virus more effectively than adults who have decreased antibody titers.64
Recognizing and Reducing Infectious Disease
As healthcare providers, optometrists should stay up to date on their own immunizations and encourage patients to do the same. Providers may consider keeping in place some or all of the general cleaning and sanitization protocols that have increased in the past year, to continue to prevent spread of any pathogen that may present to the office.
Clinicians should also be aware of local outbreaks of infectious disease to assist in differentiating ocular manifestations. In cases of red eyes or unusual presentations of ocular disease, it will become increasingly important to inquire about a patient’s immunization history and consider vaccine-preventable disease as a differential once common causes have been ruled out, particularly in cases that do not improve with standard treatment or that present with associated systemic findings.
Dr. McDowell is an associate professor at the Michigan College of Optometry, where she serves as Chief of the Pediatric and Binocular Vision Service, and is the Pediatric Residency Supervisor. She is also involved in the Pediatric Eye Disease Investigator Group (PEDIG) and chairs the Michigan Optometric Association Children’s Vision Care Committee. Dr. Williamson is a clinical assistant professor of ophthalmology at the Cleveland Clinic Lerner College of Medicine and associate staff member at the Cole Eye Institute. She is a PEDIG investigator and fellow of both the American Academy of Optometry and the Contact Lens Society of America.
1. Hartnett KP, Kite-Powell A, DeVies J, et al. Impact of the COVID-19 pandemic on emergency department visits — United States, January 1, 2019–May 30, 2020. MMWR. 2020;69(23):699–704. 2. Santoli JM, Lindley MC, DeSilva MB, et al. Effects of the COVID-19 pandemic on routine pediatric vaccine ordering and administration — United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:591–593. 3. Patel M, Lee AD, Clemmons NS, et al. National update on measles cases and outbreaks — United States, January 1–October 1, 2019. MMWR Morb Mortal Wkly Rep. 2019;68:893–896. 4. Delamater PL, Street EJ, Leslie TF, et al. Complexity of the basic reproduction number (R0). Emerging Infectious Diseases. 2019;25(1):1-4. 5. Centers for Disease Control and Prevention site. Pages for each disease listed available by index or search. www.cdc.gov 6. Ahmad M, et al. “Ocular Manifestations of Infectious Diseases” In: Levin AV, Enzenauer RW (eds) The Eye in Pediatric Systemic Disease. 327-57. Springer, 2017. 7. Issam Eddine E, Sana S, Achraf F, Chiraz A, Walid Z. Manifestations oculaires de la rougeole chez l’adulte : à propos de trois cas [Ocular manifestations of measles in adults: About three cases]. J Fr Ophtalmol. 2020;43(5):392-396. 8. Nandi A, Shet A, Behrman JR, Black MM, Bloom DE, Laxminarayan R. Anthropometric, cognitive, and schooling benefits of measles vaccination: Longitudinal cohort analysis in Ethiopia, India, and Vietnam. Vaccine. 2019;37(31):4336-4343. 9. Kutzelnigg A, Lassman H. “Pathology of multiple sclerosis and related inflammatory demyelinating diseases.” In: Goodin DS (ed). Handbook of Clinical Neurology: Multiple Sclerosis and Related Disorders. 15-58. Elsevier, 2014. 10. Kang BH, Kim JI. Opsoclonus-myoclonus syndrome associated with mumps virus infection. J Clin Neurol. 2014 Jul;10(3):272-275. 11. Babu K, De C, Sampangi R. Bilateral outer retinal necrosis following mumps infection. Ocul Immunol Inflamm. 2018;26(1):130-132. 12. Kahloun R, Ben Amor H, Ksiaa I, et al. Multimodal imaging in a case of bilateral outer retinitis associated with mumps infection. Int Ophthalmol. 2018;38(1):339-343. 13. Singh K, Sodhi PK. Mumps-induced corneal endotheliitis. Cornea. 2004;23(4):400-402. 14. Ichiba N, Miyake Y, Sato K, Oda M, Kimoto H. Mumps-induced opsoclonus-myoclonus and ataxia. Pediatr Neurol. 1988;4(4):224-227. 15. Ray S, Gragoudas E. Neuroretinitis. Int Ophthalmol Clin. 2001;41(1):83-102. 16. Kang BH, Kim JI. Opsoclonus-myoclonus syndrome associated with mumps virus infection. J Clin Neurol. 2014;10(3):272-275. 17. Onal S, Toker E. A rare ocular complication of mumps: kerato-uveitis. Ocul Immunol Inflamm. 2005;13(5):395-397. 18. Khan B, Nasir S, Hanif S. Bilateral optic neuritis: a rare complication of mumps. Cureus. 2020;12(4):e7768. 19. Irioka T, Akaza M, Nakao K, Kanouchi T, Yokota T, Mizusawa H. Chiasmal optic neuritis following mumps parotitis. J Neurol. 2008;255(5):773-774. 20. Folayan MO, Arobieke RI, Eziyi E, Oyetola EO, Elusiyan J. Facial nerve palsy: analysis of cases reported in children in a suburban hospital in Nigeria. Niger J Clin Pract. 2014;17(1):23-27. 21. de Groot-Mijnes JD, de Visser L, Rothova A, Schuller M, van Loon AM, Weersink AJ. Rubella virus is associated with Fuchs heterochromic iridocyclitis. Am J Ophthalmol. 2006;141(1):212-214. 22. Gonzales JA, Hinterwirth A, Shantha J, et al. Association of ocular inflammation and rubella virus persistence. JAMA Ophthalmol. 2019;137(4):435-438. 23. Suzuki J, Goto H, Komase K, et al. Rubella virus as a possible etiological agent of Fuchs heterochromic iridocyclitis. Graefes Arch Clin Exp Ophthalmol. 2010;248(10):1487-1491. 24. Chan NS, Chee SP. Demystifying viral anterior uveitis: A review. Clin Exp Ophthalmol. 2019;47(3):320-333. 25. Arnold J. Ocular manifestations of congenital rubella. Curr Opin Ophthalmol. 1995;6(3):45-50. 26. Pool V, Tomovici A, Johnson DR, Greenberg DP, Decker MD. Humoral immunity 10 years after booster immunization with an adolescent and adult formulation combined tetanus, diphtheria, and 5-component acellular pertussis vaccine in the USA. Vaccine. 2018 Apr 19. 36 (17):2282-2287. 27. Pichichero ME, Rennels MB, Edwards KM, et al. Combined tetanus, diphtheria, and 5-component pertussis vaccine for use in adolescents and adults. JAMA. 2005 Jun 22. 293(24):3003-11. 28. Chandler JW, Milam DF. Diphtheria corneal ulcers. Arch Ophthalmol. 1978 Jan. 96(1):53-6. 29. Coachman J. Diphtheric conjunctivitis. Mer J Ophth. 1951. 34:1176. 30. Raoof N, Pereira S, Dai S, Neutze J, Grant CC, Kelly P. Retinal haemorrhage in infants with pertussis. Arch Dis Child. 2017;102(12):1158-1160. 31. Cherry JD. Pertussis in young infants throughout the world. Clin Infect Dis. 2016;63(suppl 4):S119-S122. 32. Guggenheim JA, Williams C; UK Biobank Eye and Vision Consortium. Childhood febrile illness and the risk of myopia in UK Biobank participants. Eye (Lond). 2016;30(4):608-614. 33. Chalkia AK, Bontzos G, Spandidos DA, Detorakis ET. Human papillomavirus infection and ocular surface disease (Review). Int J Oncol. 2019;54(5):1503-1510. 34. Woods M, Chow S, Heng B, et al. Detecting human papillomavirus in ocular surface diseases. Invest Ophthalmol Vis Sci. 2013;54(13):8069-8078. 35. Hanbazazh M, Gyure KA. Ocular Human Papillomavirus Infections. Arch Pathol Lab Med. 2018;142(6):706-710. 36. Ramberg I, Sjö NC, Bonde JH, Heegaard S. Inverted papilloma of the conjunctiva. BMJ Open Ophthalmol. 2019;4(1):e000193. . 37. Rombaldi RL, Serafini EP, Mandelli J, Zimmermann E, Losquiavo KP. Perinatal transmission of human papilomavirus DNA. Virol J. 2009;6:83. 38. Kalogeropoulos C, Moschos M. Advances in diagnosis and treatment of HPV ocular surface infections. Med Hypothesis Discov Innov Ophthalmol. 2015;4(2):31-35. 39. Holt HD, Hinkle DM, Falk NS, Fraunfelder FT, Fraunfelder FW. Human papilloma virus vaccine associated uveitis. Curr Drug Saf. 2014;9(1):65-68. 40. Irani F, Ruddell T. Meningococcal conjunctivitis. Aust N Z J Ophthalmol. 1997;25(2):167-168. 41. Pickering L, Jennum P, Ibsen R, Kjellberg J. Long-term health and socioeconomic consequences of childhood and adolescent onset of meningococcal meningitis. Eur J Pediatr. 2018;177(9):1309-1315. 42. Parikh SR, Campbell H, Mandal S, Ramsay ME, Ladhani SN. Primary meningococcal conjunctivitis: summary of evidence for the clinical and public health management of cases and close contacts. J Infect. 2019;79(6):490-494. 43. Anderson J, Lind I. Characterization of Neisseria meningitidis isolates and clinical features of meningococcal conjunctivitis in ten patients. Eur J Clin Microbiol Infect Dis. 1994;13(5):388-393. 44. Dinakaran S, Chan TK, Rogers NK, Brosnahan DM. Retinal hemorrhages in meningococcal septicemia. J AAPOS. 2002;6(4):221-223. 45. Yusuf IH, Sipkova Z, Patel S, Benjamin L. Neisseria meningitidis endogenous endophthalmitis with meningitis in an immunocompetent child. Ocul Immunol Inflamm. 2014;22(5):398-402. 46. Kerkhoff FT, van der Zee A, Bergmans AM, Rothova A. Polymerase chain reaction detection of Neisseria meningitidis in the intraocular fluid of a patient with endogenous endophthalmitis but without associated meningitis. Ophthalmology. 2003;110(11):2134-2136. 47. Thornton JA, Tullos NA, Sanders ME, et al. Differential bacterial gene expression during experimental pneumococcal endophthalmitis. Ophthalmic Res. 2015;53(3):149-161. 48. Parmar P, Salman A, Kalavathy CM, Jesudasan CA, Thomas PA. Pneumococcal keratitis: a clinical profile. Clin Exp Ophthalmol. 2003;31(1):44-47. 49. Benton AH, Marquart ME. The role of pneumococcal virulence factors in ocular infectious diseases. Interdiscip Perspect Infect Dis. 2018;2018:2525173. 50. Mahajan VM, Bareja U, Prakash K, Ghose S. Pneumococci in ocular disease of children and their treatment. Ann Trop Paediatr. 1987;7(4):270-273. 51. Richardson KM, Chen KS, Goubeaux DL, et al. A 16-year-old girl with eye pain, J Ped Infect Dis Soc. 2019; 8(1): 77-79. 52. Alter SJ, Sanfilippo CM, Asbell PA, DeCory HH. Antibiotic resistance among pediatric-sourced ocular pathogens: 8-year findings from the antibiotic resistance monitoring in ocular microorganisms (ARMOR) surveillance study. Pediatr Infect Dis J. 2019;38(2):138-145. 53. Haas W, Pillar CM, Torres M, Morris TW, Sahm DF. Monitoring antibiotic resistance in ocular microorganisms: results from the antibiotic resistance monitoring in ocular microrganisms (ARMOR) 2009 surveillance study. Am J Ophthalmol. 2011;152(4):567-574.e3. 54. Wainwright M, Swan HT. C.G. Paine and the earliest surviving clinical records of penicillin therapy. Medical History. 1986;30:42-56. 55. Fishman R. The earliest success of penicillin. Am J Ophthmol. 2016; 163:204. 56. Marimon JM, Ercibengoa M, García-Arenzana JM, Alonso M, Pérez-Trallero E. Streptococcus pneumoniae ocular infections, prominent role of unencapsulated isolates in conjunctivitis. Clin Microbiol Infect. 2013;19(7):E298-E305. 57. Leung J, Broder KR, Marin M. Severe varicella in persons vaccinated with varicella vaccine (breakthrough varicella): a systematic literature review. Expert Rev Vaccines. 2017;16(4):391-400. 58. Johnston NR. Red eye in chickenpox: varicella-related acute anterior uveitis in a child. BMJ Case Rep. 2010;2010:bcr0120102678. 59. Mansour DE, El-Shazly AA, Elawamry AI, Ismail AT. Comparison of ocular findings in patients with H1N1 influenza infection versus patients receiving influenza vaccine during a pandemic. Ophthalmic Res. 2012;48(3):134-138. 60. Khairallah M, Kahloun Rim. Ocular manifestations of emerging infectious diseases, Cur Opin Ophthalmol. 2013;(24)6: 574-80. 61. Zimmerman P, Curtis N. Coronavirus infections in children including COVID-19: An overview of epidemiology, clinical features, diagnosis, treatment, and prevention options in children. Pediatr Infect Dis J. 2020;39:355-68. 62. Chen L, Deng C, Chen X, et al. Ocular manifestations and clinical characteristics of 535 cases of COVID-19 in Wuhan, China; a cross-sectional study. Acta Ophthalmol. 2020;98(8):e951-59. 63. Table adapted from Feldstein et al. Multisystem inflammatory syndrome in US children. N Engl J Med 2020;383:334-46. 64. Ludvigsson. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatrica. 2020;109:1088-95. 65. Kwok T, Al-Bermani A. Two rare cases of retinal vasculitis following vaccination. Scottish Medical Journal. 2013;58(2), e10-12. 66. Ness T, Hengel H. Impfschäden am Auge [Adverse ocular effects of vaccinations]. Ophthalmologe. 2016;113(7):615-622. 67. Fujimoto C, Shi G, Gery I. Microbial products trigger autoimmune ocular inflammation. Ophthalmic Res. 2008;40(3-4):193-99. 68. Chang MY, Pineles SL. Pediatric Optic Neuritis. Semin Pediatr Neurol. 2017;24(2):122-28. |

