Corneal vital dyes have existed in eye care since the 1880s and their use has provided us with essential information on the health of the ocular surface to help aid diagnosis and treatment. Furthermore, new drug approvals from the FDA rely on changes in ocular surface noted by corneal staining to determine side effects and endpoints of clinical trials.1
 |
|
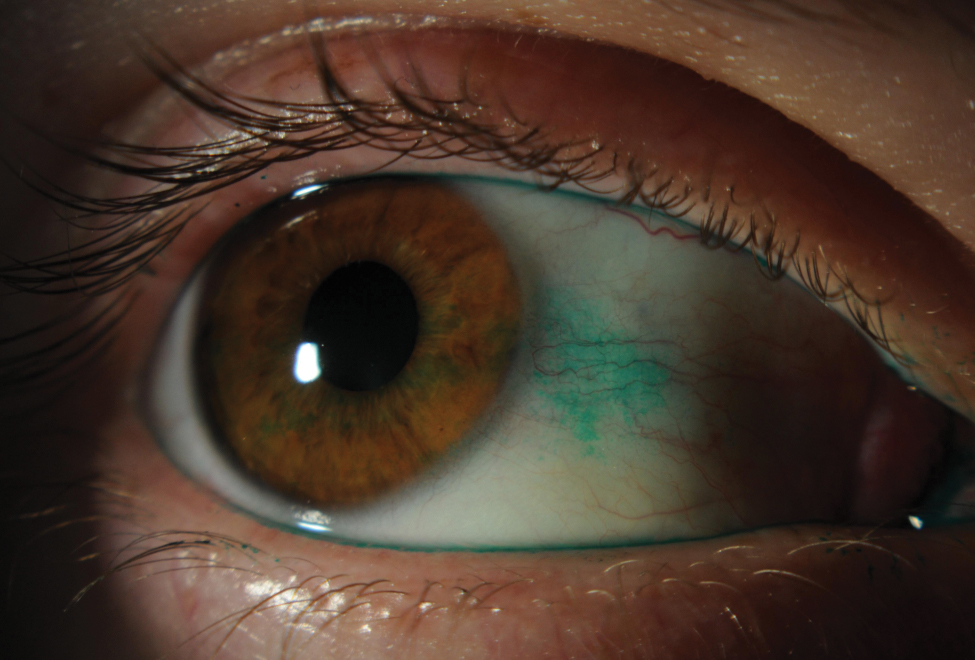
Slit lamp photo depicting lissamine green staining on conjunctiva from dry eye. Click image to enlarge. |
Vital dyes penetrate living cells or tissues without damaging them, making them diagnostically very useful. The three dyes typically used in eye care are sodium (NaFl) fluorescein, lissamine green and rose bengal, with fluorescein being the most common.2
Sodium fluorescein is an orange, water-soluble dye that penetrates damaged corneal epithelial cells. It was first used by Pfluger in 1882 when he detected positive staining on the cornea signifying breaks in the epithelium.3,4 It was then used to monitor abrasions and give insight into how the cornea heals.5,6 Since then, the use of sodium fluorescein has expanded and is now implemented as a standard in ophthalmic practice. It is used to detect and diagnose ocular conditions associated with epithelial defects, Seidel sign, Jones Dye Test nasolacrimal duct obstruction and others. It’s also used when performing applanation tonometry, evaluating tear meniscus height and fitting contact lenses fits. An intravenous form is administered when performing fluorescein angiography. A Wratten filter should be used to highlight staining when using the cobalt blue excitation filters, as it eliminates the reflected blue light.
Lissamine green is a water-soluble dye and an ideal stain for the bulbar conjunctiva, since it provides better contrast than NaFI. It stains degenerative cells where there is a disruption or damage of mucin coating.1 Since the dye diffuses into cells that are already dead and degenerative, it is not as toxic to the cornea as rose bengal and produces less irritation upon instillation.
Rose bengal is used less in practice due to its stinging and toxic effect; like lissamine green, it stains dead and devitalized cells.7,8 It has been argued that rose bengal is not actually a vital dye as it also stains healthy cells and affects their viability.9 Therefore, if herpes simplex virus (HSV) is suspected, make sure to culture prior to staining with rose bengal, as it kills the virus.10 Both lissamine green and rose bengal are better choices for assessing conjunctival staining than NaFl because it provides better contrast against the palpebral and bulbar conjunctiva.11
There have been studies where a mixture of dyes was formulated to simultaneously evaluate the cornea and conjunctiva with one drop, but no formulations are pre-mixed for purchase in general practice. The optimal concentration and mixture was either 2% or 1% fluorescein and 1% lissamine, which provided sufficient staining with the least discomfort and irritation to patients.12,13
Any of the three dyes discussed can be used in practice to determine the integrity of the health of the eye, but to understand the pathophysiology of the staining patterns, it is important to review the anatomy of the cornea and conjunctiva.
 |
|
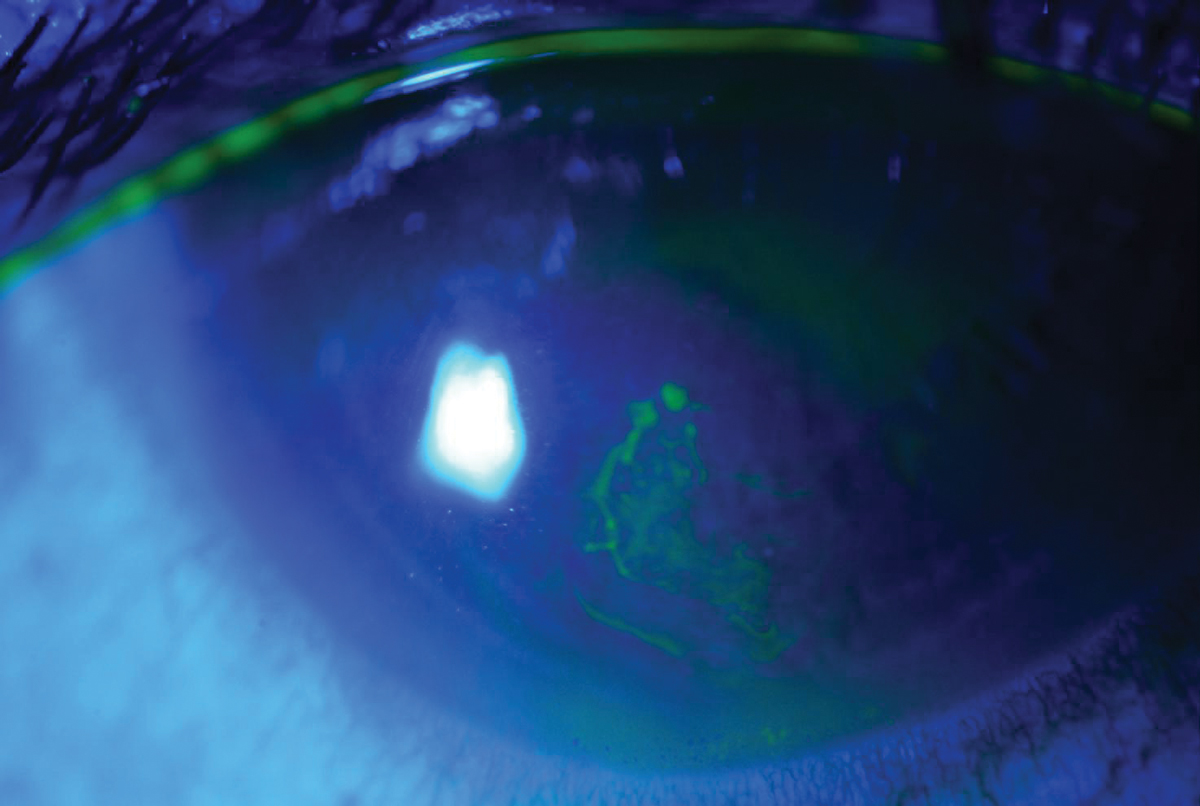
A patient with recurrent corneal erosion. Click image to enlarge. |
Cornea and Conjunctiva
The corneal epithelium is made up of non-keratinized stratified squamous epithelium, which spans across a smooth surface of five to seven layers and is about 50µm thick.14 The epithelial layers are composed of three types of cells: superficial, wing and basal cells.15 These cells are held together by desmosomes, which form tight junctions, and hemidesmosomes, which anchor the basal cells to the basement membrane. The epithelium is covered by the tear film, which is comprised of lipid, aqueous and mucin.16 The mucin layer is responsible for surface wetting, prevention of debris adhering to ocular surface and tear spreading.17
A disturbance to any area of the epithelial layers or tear layers will result in staining patterns to be evaluated and help aid in diagnosis.
The conjunctiva covers the “non-cornea” portion of the external globe and is made up of non-keratinized stratified squamous and columnar cells, which are arranged in three-to-five cell layers.18 Goblet cells interspersed in between the cells produce mucin to form part of the tear film. The conjunctiva is divided into the palpebral and bulbar regions and the fornices; it provides protection by acting as a physical barrier from foreign objects and microorganisms along with maintaining a stable tear film. Just like in the cornea, any uptake of vital staining is due to damage to the conjunctival epithelium. In healthy tissue, it is common to see the dyes accumulate in the conjunctival folds and this should be differentiated from punctate bulbar staining and mucin flecks.19
 |
|
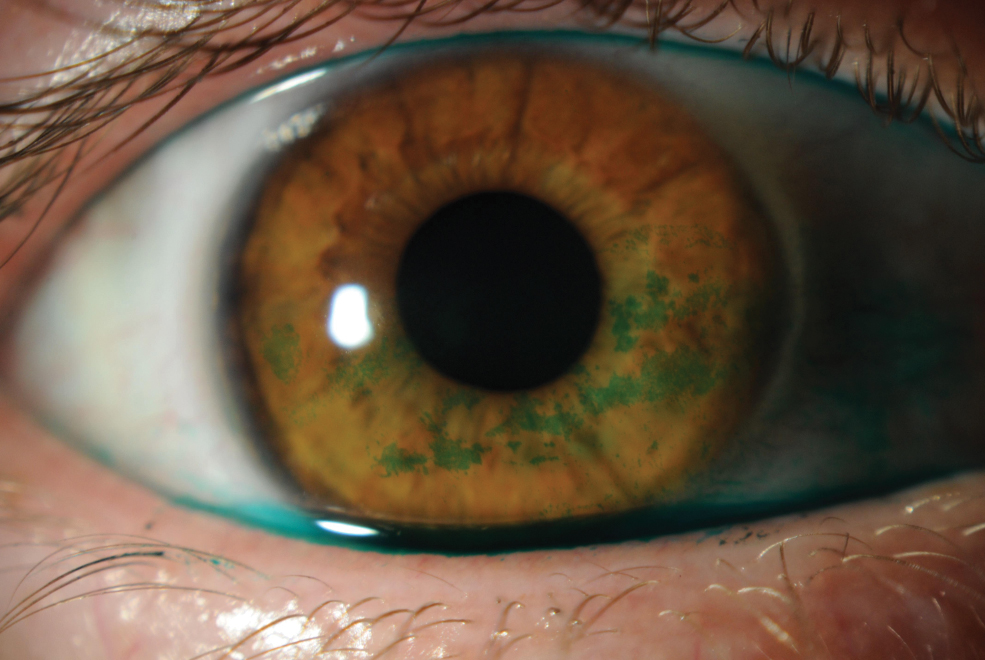
Slit lamp photo depicting lissamine green staining on cornea. Click image to enlarge. |
Staining Patterns
These can be present in various forms. The most common type is superficial punctate staining, which manifests as patterns of small dots or flecks that stain brightly with fluorescein in either a diffuse pattern or segregated patches on certain areas.20 These flecks and small stains are termed punctate epithelial erosions when focal defects are noted, or superficial punctate keratitis when focal areas of inflammation are involved. The depth, location and intensity of staining patterns are also noted to determine proper diagnosis.
There have been several studies and scales produced to grade density and severity of staining, as assessment can vary per clinician and differ based on amount of dye used and timing of observation.1 Diffuse patterns of punctate staining are mostly associated with medicamentosa or viral conjunctivitis, while specific areas such as the classic “three and nine o’clock” pattern are related to contact lens wear, causing desiccation of the peripheral cornea in areas where lids are not in direct contact with the epithelium because of the lens edge.
Not only is the location of the staining important, but so is the type of staining pattern—pooling of dye or negative staining. Negative staining represents non-stained areas where the stain has run off elevated areas of cornea, which is prominent in cases of Salzmann’s nodular degeneration and epithelial basement dystrophy.
 |
|
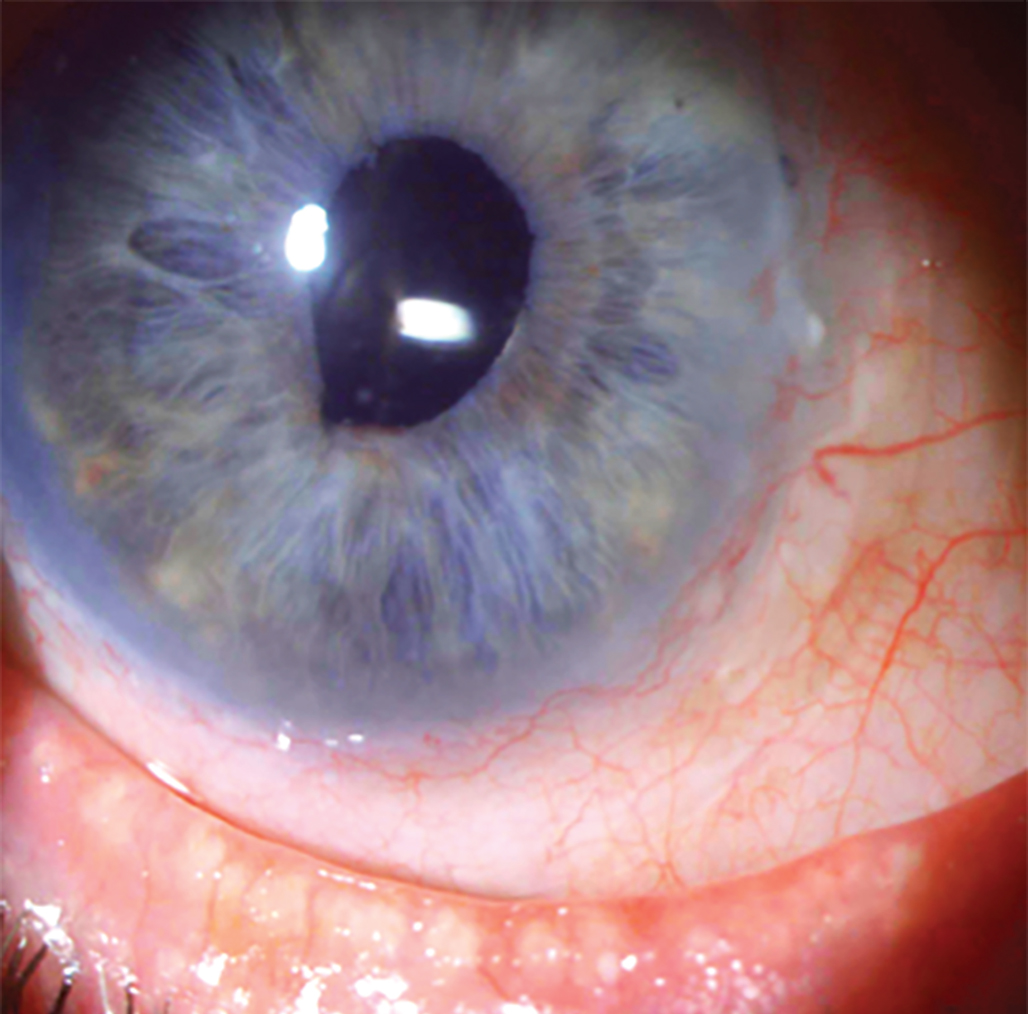
A patient with limbal stem cell deficiency. Photo: Suzanne Sherman, OD. Click image to enlarge. |
Positive staining occurs when fluorescein penetrates disruptions in the intercellular junctions of the corneal epithelial cells, causing areas of defined hyperfluoresced dots (punctate staining) that may coalesce from micro-punctate to macro-punctate or patchy patterns.21 Positive stains on the cornea are also associated with dendritic lesions as the HSV virus begins to spread to epithelial cells, causing disruption and cell lysis. In active HSV infections, the bed of the ulcerative lesion stains with fluorescein while the edges of the ulcer stain with rose bengal, so a combination of drops is useful. Positive NaFl staining is also noted in areas with complete full-thickness epithelial defects, such as ulcers or abrasions, as evidenced by diffusion of the dye into the stroma.
Pooling of dye is seen secondary to areas of corneal thinning or indentations on cornea. Some examples include pterygia, dellen and blebs. This “spillover” effect is different from actual staining and more like the effect of negative staining but over a larger area. Remember that sodium fluorescein does not penetrate where cells are intact and healthy, so the stain is filling the depressed area. Another example is called “dimple veiling,” which can be found in rigid gas permeable lens wearers with steep central clearance and a flatter peripheral curve profile, allowing small bubbles to enter and become trapped during lens wear, causing indentions and divots in the epithelium.
Cornea and Conjunctival Staining
Here are some instances of frequent patterns of corneal and conjunctival staining encountered in routine practice. Superficial superior corneal staining can range from anything that is contact lens-related to superior limbic keratopathy.22 It is typically not associated with dry eye, as the upper lid protects the superior ocular surface.
• Superior limbal keratoconjunctivitis (SLK) presents with staining and localized conjunctivochalasis produced by mechanical friction from the upper palpebral conjunctiva and superior cornea. Its presence can be associated with thyroid eye disease or possibly caused by contact lens wear.22
In thyroid eye disease, there is an association with lid retraction, known as Dalrymple’s sign, which in turn produces pressure from the upper lid, causing defective blinking and mechanical disruption to the bulbar conjunctiva. As mechanical disruption occurs, it causes tiny breaks in the cornea epithelium, resulting in small abrasions that uptake the stain.22 Therefore, on clinical examination, you may find injection on the superior bulbar conjunctiva and fine punctate staining along the superior limbus with associated filaments and neovascularization.
Corneal filaments are not only associated with SLK but also with other ocular conditions ranging from dry eye to neurotrophic keratopathy and can stain with any of the three vital dyes mentioned. They are composed of a mucin plaque covered with sloughed-off degenerative epithelial cells, lipids and protein material coalescing to create an elevated plaque.23 These can vary in size and presentation depending on the etiology. Plaques can be thin and hair-like, as seen in filamentary keratitis, or uniquely shaped as dendriform plaques, as found in herpes zoster. The base of the plaques can either be attached to the corneal epithelium or conjunctival fornix.
• Floppy eyelid syndrome is another cause of superiorly located mechanical trauma due to the laxity of the lid. The laxity is caused by loss of rigidity of the tarsal plate due to depleted levels of elastin. It is more common on the side the patient sleeps on and can include trichiasis in severe cases. This presents superficial punctate staining along with conjunctival irritation as the chronic everted eyelid or misdirected lashes rub along the cornea.24 This is a common finding in patients with keratoconus, conjunctivitis, blepharitis and obstructive sleep apnea.
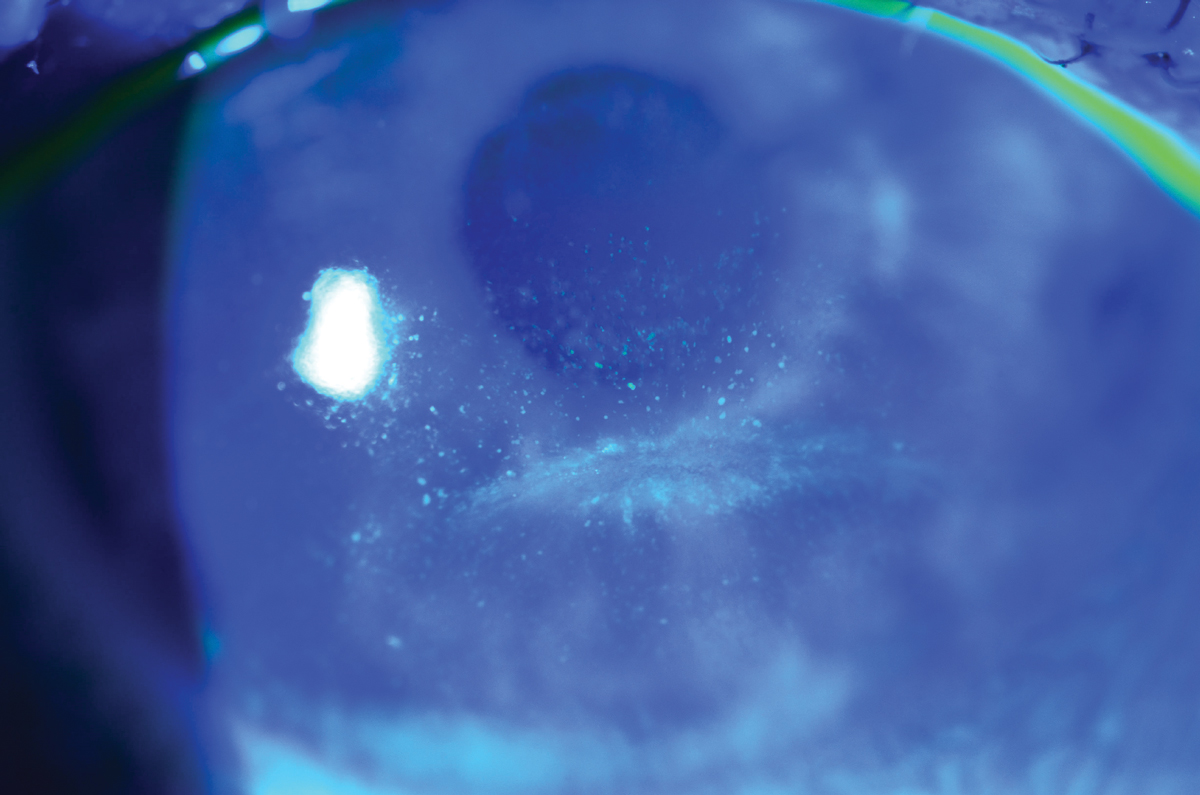 |
|
This patient with lagophthalmos from facial nerve paralysis has interpalpebral corneal staining. Photo: WR Buie, ophthalmic photographer. Click image to enlarge. |
• A foreign body under the superior lid produces linear scratches or irritation on the cornea or conjunctiva indicating breakdown of any of the three layers of the corneal epithelium and produces repeated abrasions with each blink. When any positive staining is present in a linear or curvilinear pattern, the upper lid should be everted and evaluated for any foreign bodies or misdirected lashes.
• Limbal stem cell deficiency (LSCD) results from damage or dysfunction to the limbal stem cells, whose main function is to maintain the transparency and health of the cornea.25 When there is disruption to the limbal stem cells, the conjunctival epithelium encroaches on the cornea, resulting in conjunctivalization.26,27 Fluorescein staining presents a stippled, opaque, whorl-like pattern with some cases portraying a demarcation line between the conjunctival and corneal cells.26 In the initial stages, the limbus and periphery will stain more and then progress to a more diffuse, central pattern as the severity of the disease progresses.28,29 Associations with LSCD can range from genetic conditions causing limbal stem cell dysfunction to external factors such as chronic inflammation, trauma secondary to chemical burns or surgeries or poorly fitted contact lenses.30,31
• Aqueous-deficient dry eye is most associated with interpalpebral staining and typically starts with conjunctival staining on the nasal and temporal areas, which is seen more prominently with lissamine green.31 In aqueous-deficient dry eye, there is an increase in mucin production by the goblet cells, which combines with epithelial and other debris to form filamentary plaques that will stain with rose bengal or lissamine green.
• Exposure keratopathy can occur for a variety of reasons and presents with both interpalpebral punctate staining or inferior staining, depending on the cause. Interpalpebral staining is encountered with aqueous deficiency, exposure and neurotrophic keratopathy, as there is less protection from the lids. Most interpalpebral presentations are associated with UV or chemical exposure from smoke, fumes or sunlamps from the lack of lid protection.32
Exposure keratopathy secondary to lid abnormalities or incomplete lid closure can be associated with Bell’s palsy, thyroid eye disease—which includes both Stellwag’s sign (incomplete or infrequent blinking) and Dalrymple’s sign (widened palpebral fissure)-—or floppy eyelid syndrome.33 When the tear film is disrupted, the epithelial cells are more prone to damage and suffer erosions, which will stain with sodium fluorescein in the exposed areas.
• Neurotrophic keratopathy occurs because of damage to the trigeminal nerve, causing loss of corneal sensitivity. The patient will not have complaints of irritation to match the degree of the corneal erosions noted.
The most common etiologies associated with neurotrophic keratopathy are varicella-zoster virus or HSV but the condition can also follow ocular surgery or trauma, diabetic neuropathy, topical medication use (e.g., NSAIDs, anesthetic abuse), chronic contact lens use and extensive photocoagulation. The cornea is highly innervated from the long posterior ciliary nerves and most fibers emerge from the ophthalmic branch of the trigeminal nerve. Nerves release neuromediators that protect the cornea by providing reflex blinking and nutrition. The denervation of the cornea causes dysfunction and damage in the epithelial layer because of decreased metabolism and proliferation of epithelial cells.34
• Thygeson’s superficial punctate keratitis produces small, elevated clusters of superficial and intra-epithelial corneal lesions. Lesions are centrally located and have a duller, less organized appearance than staining associated with dry eye. Lesions have an ovoid, gray-white appearance and can produce areas of negative staining with minimal to no conjunctival staining. The pathophysiology is still unknown but it is hypothesized to have a viral or immunologic mechanism without inflammatory correlations. Patients may be asymptomatic or have similar complaints to that of dry eye, including foreign body sensation, tearing or photophobia.
Inferior staining patterns are most associated with blepharitis, meibomian gland dysfunction, lid abnormalities and mechanical causes.22 Inferior staining should prompt you to evaluate the lid margin for blepharitis and meibomian gland dysfunction. A linear pattern of conjunctival and corneal staining is usually associated with blepharitis, as there is staphylococcal bacteria floating in the stagnant tear lake in the inferior conjunctival margin and irritating the cornea.
Table 1. Common Staining Patterns
| Superior | Interpalpebral | Inferior | Diffuse |
| - Superior limbal keratoconjunctivitis - Foreign body under upper lid - Atopic and vernal keratoconjunctivitis - Floppy eyelid - Conjunctival concretions - Superior entropion - Trichiasis | - Exposure keratopathy - UV keratopathy - Aqueous-deficient dry eye - Medicamentosa (diffuse) | - Blepharitis - MGD - Lagophthalmos - Ectropion - Inferior entropion - Mucus fishing syndrome - Conjunctivochalasis | - Medica-mentosa - Toxicity - Viral conjunctivitis - Bacterial |
• Conjunctivochalasis is redundant conjunctival folds that are exposed and dry and irritated. The most common cause is age-related and hypothesized to be the degradation of elastic fibers and collagen from age, mechanical factors and inflammation.35,36 There will be positive rose bengal staining on the inferior lid margin and inferior conjunctival folds, as the upper eyelids are not completely closing due to the extra conjunctival tissue and inability to evenly spread tear film.37 The folds can also cause mechanical disruption and block the punctum, leading to stagnant tear film with inflammatory cytokines, which in turn can cause ocular surface inflammation and linear staining on the mucosal area of the lid margin.38 This is seen mostly on the inferior temporal bulbar conjunctiva, with breaks in the tear meniscus with staining as the tear film is destabilized by redundant folds.
Mechanical factors can also be associated with inferior staining patterns, particularly in cases of mucus fishing syndrome. In such patients, there will be prominent lissamine green uptake in the inferior bulbar conjunctival and nasal fornices due to constant mechanical irritation from the eye. This usually stems from foreign body sensation associated with dry eye, causing eye rubbing and removal of mucus strands formed from epithelial injury. The activity typically continues in an ongoing cycle with more mucin being produced as the patient “fishes out” the mucus strands.
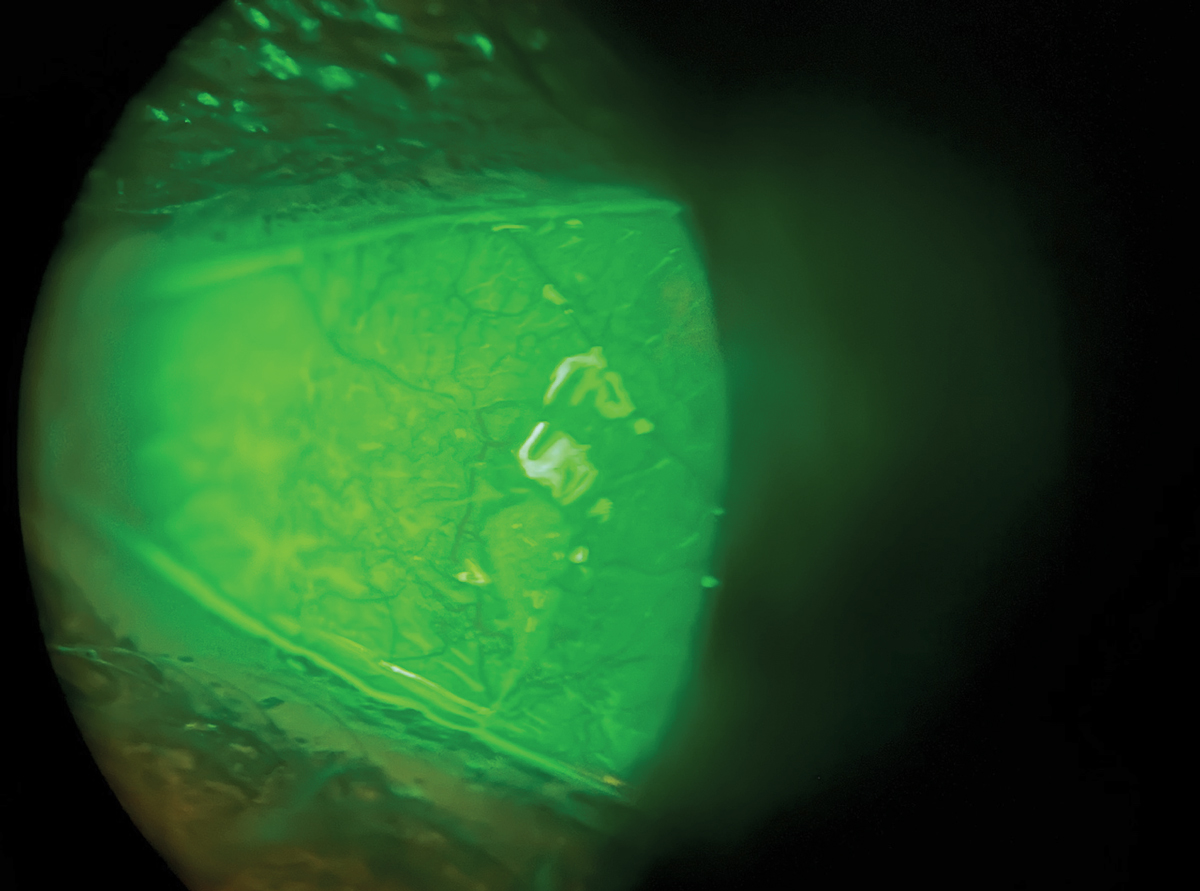 |
|
Fluorescein staining seen in dry eye. Photo: Milton Hom, OD. Click image to enlarge. |
• Lid wiper epitheliopathy presents with staining on the superior and inferior lid margins.39 The Marx line is a clear boundary that runs along the inner surfaces of the superior and inferior eyelid margin at the mucocutaneous junction.40 This line connects the area where meibomian glands secrete lipids and the aqueous tear fluid.40 Positive lissamine staining highlights its positioning and irregularity, which can be an indication of gland dysfunction. The position can shift outside the lid margin area decreasing the friction as the lids become more lax with aging or can be more intense due to added friction on lids from contact lenses.41
Lid wiper epitheliopathy is caused by damage to the epithelial cells in the Marx line region associated with frictional forces of each blink and tear film instability.42 This is an additional area to focus on that will give insight on patients complaining of dry eye symptoms with no other clinical signs prior to seeing corneal staining.
Takeaways
Dry eye is one of the most common diagnoses encountered in routine exams and understanding the pathophysiology of vital dyes and their staining patterns will result in appropriate management and patient education. It’s important to note that staining patterns not only provide insight, but also can be associated with a combination of causes (e.g., atopic keratoconjunctivitis and mucus fishing syndrome).
The health of the ocular surface is vital for clear vision, so when a patient has symptoms and no other obvious cause, reaching for vital dyes and exploring the patterns noted on the conjunctiva and cornea is an important tool to get to the result and find the most suitable treatment plan.
Dr. Rojas is an instructor in optometric science (in Ophthalmology) at Columbia University Medical Center. She received her undergraduate degree from the University of Central Florida and graduated from Nova Southeastern College of Optometry. She completed her optometric residency in ocular disease at SUNY College of Optometry. She specializes in medically necessary contact lens fittings and dry eye, and comanages refractive, corneal and ocular disease. She has no financial disclosures.
1. Begley C, Caffery B, Chalmers R, et al. Review and analysis of grading scales for ocular surface staining. The Ocular Surface. 2019;17(2):208-20. 2. Wu Y, Wang C, Wang X, et al. Advances in dry eye disease examination techniques. Front Med. 2021;8:826530. 3. Morgan P, Maldonado-Codina C. Corneal staining: Do we really understand what we are seeing? Cont Lens Anterior Eye. 2009;32(2):48-54. 4. Eliason JA, Maurice DM. Staining of the conjunctiva and conjunctival tear film. Brit Jour Ophthal. 1990;74:519-22. 5. Ward KW. Superficial punctate fluorescein staining of the ocular surface. Optom Vis Sci. 2008;85(1):8-16. 6. Romanchuk KG. Fluorescein. Physicochemical factors affecting its fluorescence. Surv Ophthalmol. 1982;26:269-83. 7. Kim J, Foulks GN. Evaluation of the effect of lissamine green and rose bengal on human corneal epithelial cells. Cornea. 1999;18(3):328-32. 8. Efron N. Putting vital stains in context. Clinical and Experimental Optometry. 2012;96(4):400-21. 9. Seitzman GD, Cevallos V, Margolis TP. Rose bengal and lissamine green inhibit detection of herpes simplex virus by PCR. Am J Ophthalmol. 2006 Apr;141(4):756-8. 10. Stroop WG, Chen TM, Chodosh J, et al. PCR assessment of HSV-1 corneal infection in animals treated with rose bengal and lissamine green B. Invest Ophthalmol Vis Sci. 2000;41(8):2096-102. 11. McDonnell C, Murphy O, Cochrane, A, et al. Lissamine green - where have we been and where are we now? Optometry in Practice. 2020;21(2):C-75784. 12. Korb DR, Herman JP, Finnemore VM. An evaluation of the efficacy of fluorescein, rose bengal, lissamine green, and a new dye mixture for ocular surface staining. Eye Contact Lens. 2008;34(1):61-4. 13. Yoon KC, Im SK, Kim HG, You IC. Usefulness of double vital staining with 1% fluorescein and 1% lissamine green in patients with dry eye syndrome. Cornea. 2011;30(9):972-6. 14. DelMonte DW, Kim T. Anatomy and physiology of the cornea. Journal of Cataract & Refractive Surgery. 2011;37(3): 588-98. 15. Sridhar MS. Anatomy of cornea and ocular surface. Indian J Ophthalmol. 2018; 66(2):190-4. 16. Kanski JJ, Menon J. Clinical Ophthalmology. Butterworth-Heinemann. 2003. 17. Willcox MDP, Argüeso P, Georgiev GA, et al. TFOS DEWS II Tear Film Report. Ocular Surf. 2017;15(3),366-403. (17) 18. Downie LE, Banditz S, Bergmanson JPG, et al. BCLA Clear - anatomy and physiology of the anterior eye. Cont Lens Ant Eye. 2021;44(2):132-56. 19. Bron AJ, Argüeso P, Irkec M, Bright FV. Clinical staining of the ocular surface: mechanisms and interpretations. Prog Retin Eye Res. 2015;44:36-61. 20. Bandamwar KL, Papas EB, Garrett Q. Fluorescein staining and physiological state of corneal epithelial cells. Cont Lens Ant Eye. 2014;37(3):213-23. 21. Komai S, Yokoi N, Kato H, et al. Clinical implication of patchy pattern corneal staining in dry eye disease. Diagnostics. 2021;11(2):232. 22. Fennger BJ, Tong J. Corneal staining characteristics in limited zones compared with whole cornea documentation for the detection of dry eye subtypes. Invest Ophthalmol Vis Sci. 2013;54:8013-9. 23. Fraunfelder FT, Wright P, Tripathi RC. Corneal mucus plaques. Am J Ophthalmol. 1977;83:191-7. 24. Alsuhaibani A, Mudhaiyan T. Floppy eyelid syndrome. EyeWiki. American Academy of Ophthalmology. October 21, 2021. Accessed June 27, 2022. www.eyewiki.aao.org/floppy_eyelid_syndrome. 25. Le Q, Xu J, Deng SX. The diagnosis of limbal stem cell deficiency. Ocular Surf. 2018;16(1):58-69. 26. Dua HS. Stem cells of the ocular surface: scientific principles and clinical applications. Br J Ophthalmol. 1995;79:968-9. 27. Tseng SC. Significant impact of limbal epithelial stem cells. Indian J Ophthalmol 2000;48:79-81. 28. Dua HS, Saini JS, Azuara-Blanco A, Gupta P. Limbal stem cell deficiency: concept, aetiology, clinical presentation, diagnosis and management. Indian J Ophthalmol. 2000;48:83-92. 29. Vora GK, Daluvoy MB. Diagnosis and Management of Limbal Stem Cell Deficiency. Ophthalmic Pearls. February 2014. Accessed June 27, 2022. www.aao.org/assets/a87b735e-0790-4801-a864-5cccfb43890b/635559897712770000/february-2014-ophthalmic-pearls-pdf. 30. Deng SX, Borderie V, Chan CC, et al. Global consensus on definition, classification, diagnosis and staging of limbal stem cell deficiency. Cornea. 2019;38(3):364-75. 31. Uchiyama E, Aronowicz JD, Butovich IA, McCulley JP. Pattern of vital staining and its correlation with aqueous tear deficiency and meibomian gland dropout. Eye Contact Lens. 2007;33(4):177-9. 32. Singh P, Tripathy K. Keratopathy. StatPearls. Treasure Island (FL): StatPearls Publishing. February 21, 2022. 33. Fu L, Patel BC. Lagophthalmos. StatPearls. Treasure Island (FL): StatPearls Publishing. November 2, 2021. 34. Jabbour S, Ashton C, Bala S, et al. The management of neurotrophic keratitis. Current Opinion in Ophthalmology. 2021;32(4):362-8. 35. Meller D, Tseng SC. Conjunctivochalasis: literature review and possible pathophysiology. Surv Ophthalmol. 1998;43(3):225-32. 36. Marmalidou A, Kheirkhah A, Dana R. Conjunctivochalasis: a systematic review. Surv Ophthalmol. 2018;63(4):554-64. 37. Di Pascuale MA, Espana EM, Kawakita T, Tseng SCG. Clinical characteristics of conjunctivochalasis with or without aqueous tear deficiency. Br J Ophthalmol. 2004;88(3):388-92. 38. Yvon C, Patel BC, Malhotra R. Conjunctivochalasis. StatPearls. Treasure Island (FL): StatPearls Publishing. March 12, 2022. 39. Yamaguchi M, Kutsuna M, Uno T, et al. Marx line: fluorescein staining line on the inner lid as indicator of meibomian gland function. Am J Ophthalmol. 2006;141(4):669-75. 40. Efron N, Brennan NA, Morgan PB, Wilson T. Lid wiper epitheliopathy. Prog Retin Eye Res. 2016;53:140-74. 41. Norn M. Meibomian orifices and Marx’s line. Studied by triple vital staining. Acta Ophthalmol (Copenh). 1985;63(6):698-700. 42. Knop E, Knop N, Zhivov A, et al. The lid wiper and muco-cutaneous junction anatomy of the human eyelid margins: an in vivo confocal and histological study. J Anat. 2011;218(4):449-61 |

