 A 70-year-old white male presented to the office as a new patient in March 2008 with complaints of blurred vision and glare, especially in the evening.
A 70-year-old white male presented to the office as a new patient in March 2008 with complaints of blurred vision and glare, especially in the evening.
The blur and glare had progressed gradually over the past six months. He was taking no medications and reported no drug allergies. The patient reported that his brother has glaucoma.
Diagnostic Data
Entering visual acuity through his current spectacle prescription was 20/25-2 O.D. and O.S. Pupils were equal, round and reactive to light, with no afferent pupillary defect. Extraocular motilities were full in all positions of gaze.
Best-corrected visual acuities through hyperopic astigmatic and presbyopic correction were 20/20 O.D. and O.S., and 20/15 O.U. A slit lamp examination of his anterior segments was unremarkable. Intraocular pressures at noon were 21mm Hg O.D. and O.S.
Through dilated pupils, his crystalline lenses were clear except for a few vacuoles in both eyes.
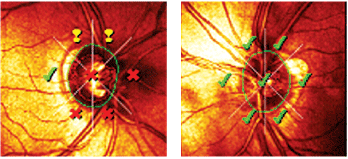
Stereoscopic evaluation of his optic nerves demonstrated cup-to-disc ratios of 0.80 x 0.85 O.D. and 0.30 x 0.40 O.S. The right neuroretinal rim was thinned from 5 to 11 oclock. His macular evaluations were normal O.U. He had bilateral posterior vitreous detachments. His peripheral retinal evaluations were unremarkable.
Given the optic nerve appearance of significant asymmetry in the
neuroretinal rims, we asked the patient to return for a full glaucoma work-up.
At his follow-up visit eight weeks later, his IOPs were 19mm Hg O.D. and 18mm Hg O.S. Pachymetry readings were 591m O.D. and 600m O.S. Threshold visual fields were completely normal O.D. and O.S.
Gonioscopy demonstrated grade 2 to grade 3 open angles in both eyes with minimal trabecular pigmentation. There were no angle abnormalities. Heidelberg Retinal Tomograph-3 (HRT-3) imaging of both optic nerves confirmed the asymmetry of the neuroretinal rims, and Moorfields Regression Analysis confirmed a right optic nerve outside normal parameters. (The left optic nerve was unremarkable.) The nerve fiber layer surrounding the right optic nerve was depressed compared to that of the left, and both optic nerves were of average size.
Given his thicker pachymetry measurements, his IOPs in the upper teens, and significant optic nerve cupping O.D., it appears that this is a straightforward case of normotensive glaucoma O.D., and that the patient should start therapy to stave off further nerve damage in the right eye. Or is it so straightforward? We need to answer some important questions before confirming this diagnosis.
Heidelberg Retinal Tomograph-3 imaging confirmed the asymmetry of the neuroretinal rims (O.D. left, O.S. right). Moorfields Regression Analysis demonstrated that the right optic nerve was outside of normal parameters.
Discussion
When initially evaluating patients to determine the risk or the presence of glaucoma, keep in mind that youre seeing the patient at one particular point in time. The findings from that visit are essentially a snapshot of their status at that moment in time. For patients who are converting to glaucoma or progressing in the disease, and for those who are poorly managed or otherwise unstable, this snapshot of findings will change with subsequent visits. Typically, that change manifests as a change in IOP, visual fields or optic nerve morphology.
In this case, how do we know that the patients right optic nerve is actually undergoing change? Some change has likely occurred to the right optic nerve. It would be exceedingly rare if such a large asymmetry between the right and left optic nerves were an anatomical, and otherwise normal, variation. Also, both optic nerves are the same size, which makes the possibility of anatomical variation even that much more remote. Certainly, if the right optic nerve was substantially larger than the left optic nerve, you could have a large asymmetry in the cup-to-disc ratio. But thats simply not the case here.
We can assume that a change has occurred to his right optic nerve, but the big question is: When did it occur? While that may sound inconsequential, it really matters in this situation.
What if his right optic nerve is not currently undergoing change, but instead what were seeing is a manifestation of something that occurred years ago? We need to answer this question before we determine whether he has an active disease process or something thats the result of a previous event. If he has an active process, such as normotensive glaucoma, he needs to be actively managed. But, if he has a manifestation of a previous event, then he simply needs to be monitored.
What type of event, not glaucoma related, is most likely to cause such an asymmetry? In patients with significant optic nerve asymmetry, especially in the context of pressure-independent glaucoma, be sure to ask the patient whether he or she has ever sustained significant loss of blood. Such events likely occur following significant trauma, such as motor vehicle accidents, or following surgical procedures where blood flow is significantly reduced.
In cases of significantly decreased perfusion, especially to the head, one or both optic nerves can become compromised due to ischemic causes. This results in damage to the optic nerve, which may be visible by ophthalmoscopy. More often than not, these patients have decreased vision, variable visual field loss and an afferent pupillary defect. Advanced stages of normotensive glaucoma may involve decreased vision and visual field defects, but the afferent pupillary defect is usually last to develop.
Patients who have undergone experiences, such as trauma or surgery, that cause significant interruption of the blood flow to the optic nerves present with findings consistent with ischemic optic neuropathy (ION)either anterior non-arteritic ION (NAION) or posterior ION. The subtle, but significant, differences between ION and glaucomatous optic neuropathy can help you to determine which situation exists.
For instance, optic nerve cupping with loss of neuroretinal rim tissue can occur in both conditions. But, ION is often accompanied by pallor of the optic nerve, whereas glaucomatous optic neuropathy usually is not. Increased optic nerve cupping and neuroretinal rim loss is also found in arteritic ischemic optic neuropathy (AION).1
The visual fields of ION nerves also differ. Typically, ION field loss is altitudinal or cecocentral in nature, whereas glaucomatous optic neuropathy typically results in arcuate field defects. Most importantly, NAION typically results in an afferent pupillary defect, whereas glaucoma rarely does.
The clinical picture can be further clouded by other concurrent findings, such as pseudophakic optic nerve pallor, in which case a glaucomatous nerve would have a pale neuroretinal rim, or in cases of bilateral NAION, in which there would be no afferent pupillary defect.
While the clinical findings of NAION and glaucomatous optic neuropathy can be differentiated at the slit lamp, questioning the patient can also help you to determine the etiology of the asymmetric optic nerve cupping. Asking about significant trauma (motor vehicle accidents, internal injuries resulting in internal bleeding, etc.) or major surgical procedures (aortic aneurysm repair, coronary artery bypass grafts, etc.) can yield suspicion of hypovolemia as a possible etiology. Many surgical procedures can result in hypovolemic optic neuropathy, including blepharoplasty.2-5
As for this patient, he did not have a history of significant trauma or major surgery with acute vision changes. He had no optic nerve pallor, no visual field defect associated with NAION and no afferent pupillary defect. So, by process of elimination, and excluding other potential causes of asymmetric neuroretinal rim loss, I diagnosed normotensive glaucoma O.D.
I prescribed Travatan Z (travoprost, Alcon), O.D. h.s. At two subsequent follow-up visits, his IOP was reduced to 10mm Hg to 11mm Hg O.D. I am currently evaluating his overall stability and control.
1. Hayreh SS, Jonas JB. Optic disc morphology after arteritic anterior ischemic optic neuropathy. Ophthalmology 2001 Sep;108(9):1586-94.
2. Suzuki D, Ilsen PF. Hypovolemic ischemic optic neuropathy. Optometry 2000 Aug;71(8):501-10.
3. Sadaba LM, Garcia-Layana A, Maldonado MJ, Berian JM. Bilateral ischemic optic neuropathy after transurethral prostatic resection: a case report. BMC Ophthalmol 2006 Oct 11;6:32.
4. Gaillard MC, Zambaz BD, Borruat FX. Posterior ischemic optic neuropathy: case report of a rare complication after general surgery. Klin Monatsbl Augenheilkd 2004 May;221(5):421-3.
5. Kordic H, Flammer J, Mironow A, Killer HE. Perioperative posterior ischemic optic neuropathy as a rare complication of blepharoplasty. Ophthalmologica 2005 May-Jun;219(3):185-8.

