 |
Infectious keratitis is a common condition that carries a high risk of visual morbidity. Often, these patients present with a painful red eye and varying vision loss following an injury to the cornea or, perhaps, a contact lens related mishap. Epithelial disruption and focal stromal infiltration with edema are hallmark findings of infectious keratitis.
Optometrists and comprehensive ophthalmologists have grown accustomed to success with fluoroquinolone antibiotics in managing mild and moderate cases. Unfortunately, things don’t always go according to plan, even with these superior antibiotics. Patterns of bacterial resistance have begun to develop.
While bacteria are the most common cause of infectious keratitis, fungal and protozoan infections do occur, and delays in diagnosis often result in a poorer visual outcome, especially if topical corticosteroids are used adjunctively with an ineffective antibiotic. In the past several years, one of the most common causes of malpractice litigation against optometrists that we have seen is alleged mismanagement of infectious keratitis.
There are various approaches to managing suspected bacterial keratitis. Some use fluoroquinolones, while others use fortified antibiotics. Some rely on topical steroid adjuncts, while others opt for no steroids. While some use microbiologic studies, others employ empiric therapy.
Of course, few can argue against a good outcome. When the outcome is poor, such as in the case of resistant bacteria, or a fungal or protozoan cause, litigation becomes a possibility.
In cases where we have defended our colleagues in these situations, invariably the plaintiff’s attorney will ask at some point in the deposition, “where are the culture results?”
In this column, we look at practical management of infectious keratitis.
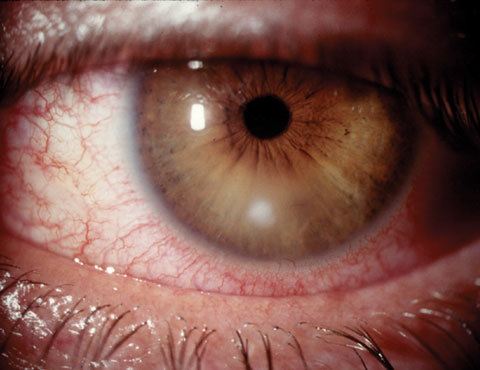 |
| Small noncentral corneal ulcers such as this are typically treated successfully empirically, while larger or more sight-threatening lesions may require culturing. Click image to enlarge. |
Treatment Methods
Historically, bacterial keratitis management involved the combined use of two fortified antibiotics, usually an alternating regimen of cefazolin 10% and an aminoglycoside, such as tobramycin 1.5%. These medications are developed from parenteral forms in a compounding pharmacy and used off-label. They can be challenging to obtain, have a limited shelf life and can be quite corneotoxic.
Unquestionably we have become comfortable with the success of the newer fluoroquinolone antibiotics. Many have replaced fortified antibiotic use with later generation fluoroquinolones due to their availability, tolerability and effectiveness. In a study comparing gatifloxacin 0.3% with fortified tobramycin and cefazolin in treating bacterial keratitis, investigators report that fluoroquinolone monotherapy was equivalent to fortified combination therapy.1 A similar study individually comparing both moxifloxacin 0.5% and gatifloxacin 0.3% with combined fortified cefazolin and tobramycin in bacterial corneal ulcers ranging in size from 2mm to 8mm found no difference in clinical cure rates, with fluoroquinolone monotherapy performing as successfully as fortified polytherapy.2 Moxifloxacin 0.5% was also seen in another report to have the same healing success as fortified polytherapy.3 While no data from prospective, controlled, human clinical trials is available regarding the specific use of besifloxacin for bacterial keratitis, several publications advocate for this agent as a safe and effective therapy.4,5
In the Literature
The success of later generation fluoroquinolone monotherapy has changed how practitioners approach cases of suspected bacterial keratitis. A report surveying ophthalmologists in a four-state area found that most respondents initiate empiric therapy with the newer fluoroquinolone antibiotics for corneal ulcers, forgoing Gram staining and culturing.6
In another survey, a minority of corneal ulcers were Gram stained or cultured, though cornea specialists were more likely to perform both. The most popular antibiotic for the treatment of less severe ulcers was moxifloxacin, while the most popular treatment of more severe ulcers was a fortified broad-spectrum antibiotic. Cornea specialists were more likely than noncornea specialists to prescribe fortified antibiotics instead of later generation fluoroquinolones for more severe corneal ulcers.7
Table 1. Culture Testing Options | |
Despite longstanding recommendations to stain and culture cases of presumed infectious keratitis, approximately half of all comprehensive ophthalmologists have long forgone microbiologic study in favor of empiric treatment.8
Formal microbiologic evaluation of microbial keratitis involves multiple corneal scrapings for samples to culture on various growth media such as chocolate agar, 5% sheep blood agar with Columbia agar base (SBA), Gram stain, Sabouraud agar, thioglycolate broth and brain heart infusion broth.9 This culturing method increases the probability of recovering a responsible pathogen from corneal tissue, which has a relatively low microbial load. It is not, however, cost effective for most eye care practitioners.
Obtaining and maintaining fresh, unexpired media, storing and transporting it properly is costly and can be a deterrent for many.
Other Methods
An alternative to directly plating corneal scrapings involves commercially available swab systems that employ a transport medium designed to keep organisms viable until a lab can inoculate appropriate media. With this method, a rayon swab is rolled or rubbed across the infiltrate in order to collect organisms. It is then placed into a tube containing transport media such as Amies agar gel or modified Stuart’s medium. The transport medium presumably now carrying the organism is then sent to a lab and plated onto the appropriate substrates.
One such commercially available device is the BD CultureSwab (Becton-Dickinson).10 Another is the ESwab (Copan Diagnostics). Whichever brand you choose, the nylon-tipped swab uses spray-on flocked fiber technology, improving sample collection and specimen release, with less entrapment than dacryon, rayon and cotton tips. The swab uses modified Amies medium, which maintains sample viability for 48 hours and has a shelf-life of 18 months.9 These swab-based transport media are inexpensive and an account with a local lab can easily be set up to facilitate pick up of specimens with subsequent analysis.
Of course, it is incumbent upon the practitioner to know which cultures or other tests are standard for their given lab, and which additional tests may be pertinent to the case, such as a fungal culture or antimicrobial sensitivities. Table 1 lists some of the more common options for corneal specimens.
Despite their ease of use, these devices have been historically avoided due to a perception of ineffectiveness and low recovery or organisms in a condition that typically yields extremely few inocula even when using corneal scrapings. However, these fears may be unfounded. Investigators report transport media using these commercially available kits are quite successful in identifying infectious organisms in cases of keratitis, in some cases matching that seen with traditional culturing methods.9-11
Even with the ease and effectiveness of the simplified microbiological specimen collection devices, many cases of suspected infectious keratitis will still be treated empirically. Most do not advocate microbiologic study on every case of suspected infectious keratitis, but those with the greatest potential for vision loss should be considered for evaluation. A guide to identifying ulcers at risk of vision loss is the “1, 2, 3” rule. To identify potentially sight-threatening ulcers, any one of the following characteristics must be present:
1. > Cells 1+ in the anterior chamber (10 cells or greater in 1mm beam);
2. Dense infiltrate greater than 2mm in greatest linear dimension (by slit-lamp light measurement);
3. Edge of infiltrate smaller than 3mm from the center of cornea.12
Infectious keratitis is a potentially sight-threatening issue with several diagnostic and therapeutic approaches. Commercially available later generation fluoroquinolones and one-step culturing devices can help obtain a successful outcome for patients.
|
1. Sharma N, Arora T, Jain V, et al. Gatifloxacin 0.3% versus fortified tobramycin-cefazolin in treating nonperforated bacterial corneal ulcers: randomized, controlled trial. Cornea. 2016;35(1):56-61. 2. Shah V, Tandon R, Satpathy G, et al. Randomized clinical study for comparative evaluation of fourth-generation fluoroquinolones with the combination of fortified antibiotics in the treatment of bacterial corneal ulcers. Cornea. 2010;29(7):751-7. 3. Sharma N, Goel M, Bansal S, et al. Evaluation of moxifloxacin 0.5% in treatment of nonperforated bacterial corneal ulcers: a randomized controlled trial. Ophthalmology. 2013;120(6):1173-8. 4. Mah F, Sanfilippo C. Besifloxacin: efficacy and safety in treatment and prevention of ocular bacterial infections. Ophthalmol Ther. 2016 June;5(1):1–20. 5. Schechter B, Parekh J, Trattler W. Besifloxacin ophthalmic suspension 0.6% in the treatment of bacterial keratitis: a retrospective safety surveillance study. J Ocul Pharmacol Ther. 2015 Mar;31(2):114-21. 6. Hsu H, Nacke R, Song J, et al. Community opinions in the management of corneal ulcers and ophthalmic antibiotics: a survey of four states. Eye Contact Lens. 2010;36(4):195-200. 7. Park J, Lee K, Zhou H, et al. Community practice patterns for bacterial corneal ulcer evaluation and treatment. Eye Contact Lens. 2015;41(1):12-8. 8. McDonnell P, Nobe J, Gauderman W, et al. Community care of corneal ulcers. Am J Ophthalmol. 1992;114(5):531-8. 9. Pakzad-Vaezi K, Levasseur S, Schendel S, et al. The corneal ulcer one-touch study: a simplified microbiological specimen collection method. Am J Ophthalmol. 2015;159(1):37-43. 10. Epley K, Katz H, Herling I, Lasky J. Platinum spatula versus mini-tip culturette in culturing bacterial keratitis. Cornea. 1998;17(1):74-8. 11. McLeod S, Kumar A, Cevallos V, et al. Reliability of transport medium in the laboratory evaluation of corneal ulcers. Am J Ophthalmol. 2005;140(6):1027-31. 12. Vital M, Belloso M, Prager T, Lanier J. Classifying the severity of corneal ulcers by using the “1, 2, 3” rule. Cornea. 2007;26(1):16-20. |

