Every day we are confronted with patients who experience discomfort while wearing contact lenses. This often manifests as non-specific complaints of dryness, burning, stinging or signs of redness and blur. Traditionally, optometry has asserted that dryness is a relative contraindication for wearing contact lenses, with the exception of fitting scleral lenses for cases of Sjögren’s, cicatricial pemphigoid or other disease-related desiccation of the ocular surface. But as technology develops, we must re-evaluate this wisdom and ask: Can patients experiencing dryness today wear contact lenses?
Dry Eye Discomfort
The International Dry Eye Workshop (DEWS) report is currently being updated. One of the myriad details emerging from the 2007 report was a discussion about the role contact lens wear can play in both aqueous tear-deficient and evaporative dry eye.1 The report exhaustively reviewed the subject of contact lens symptomatology, finding that 50% of contact lens wearers report dry eye symptoms.1 They are 12 times more likely than emmetropes and five times more likely than spectacle wearers to report dry eye symptoms.1
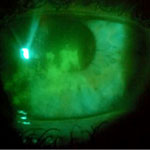
| Figure 1a: Clinical finding of poor tear film stability caused by meibomian dysfunction, which results in the lipid layer thinning (arrow). | |
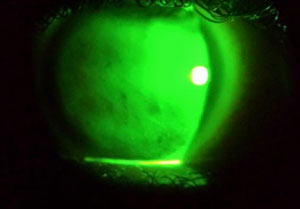 | |
| Figure 1b: Poor surface wetting after lens removal. |
As is true of dry eye complaints in the non-contact lens wearing population, female wearers reported symptoms at a rate roughly 50% higher than men, suggesting a hormonal influence.1 Associated factors cited were loss of corneal sensitivity, increased tear osmolarity, trigeminal denervation, pre-lens tear film thinning (particularly of the lipid layer), higher water content contact lenses and shortened blink intervals.1
Various investigators estimate the number of contact lens wearers who “drop out” due to discomfort (including symptoms of dryness) at 12% to 51%, depending on how you define “drop out.”2 Drop outs have been variously defined as those whose discomfort results in reduced wearing time, intermittent wear time, temporary discontinuance of wear over varying periods and permanent discontinuation of contact lens wear.2 This study reaffirms the considerable overlap between the two distinctly different yet intertwined conditions.2
Another important publication was the International Workshop on Meibomian Gland Dysfunction (MGD).3 This compendium reviews what is known about MGD, including discussions related to the interactions between contact lenses and lipids, and their contributory role in MGD. Important takeaways include: a recognition of complex differences in lipid attraction among the different FDA groups of polymers; increased instability of the tear film is caused by the presence of a contact lens, leading to evaporative drying; and these processes have associated higher rates of discomfort or intolerance.3-6
Evaluating the Problem
The old adage, “seek and ye shall find” applies here. Not all patients with dry eye disease are symptomatic, nor do the clinical signs correlate well with the symptoms.6,7 For this reason, it is imperative we not only thoroughly review the history of a patient’s contact lens use, but also probe for symptoms and signs of dry eye (Table 1).
| Table 1: Historical points, common signs and symptoms associated with contact lens discomfort and dryness. | |||
| Historical Points | Symptoms | Signs | |
| Average wear time | Dryness | Pre-lens tear film thinning and debris | |
| Overnight wear | Scratchiness | Reduced tear prism height | |
| Replacement interval | Watering | Poor lens wetting | |
| Overall compliance | Blurry vision | MGD | |
| Care system | Photophobia | Bulbar and limbal hyperemia | |
| Hygiene of lens | Stinging | Cornea and conjunctival staining | |
| Hygiene of case | Burning | Lid parallel conjunctival folds | |
| Environmental factors | Tiredness | Lid-wiper epitheliopathy | |
| Allergies | Itchiness | ||
| Pain | |||
History and symptoms are relatively easy to elicit with appropriate questioning. Assessing the signs, though, can be a little more daunting, given their relatively poor individual associations with dry eye. Experts argue the relative merits of one test over another, but research shows the most effective approach is one that uses a combination of both patient symptoms and clinical signs.1
The approach discussed below is evidence-based, employing some of the stronger clinical markers—i.e., symptoms, meibomian gland dysfunction, lid wiper epitheliopathy (LWE), lid-parallel conjunctival folds (LIPCOFs)—and associated symptoms of contact lens discomfort. This is certainly not an inclusive discussion and should be modified as your judgment or the emerging science dictates.
1. Ask the patient. Multiple questionnaires are available to determine whether or not a patient is symptomatic, such as the Ocular Surface Disease Index, Contact Lens Dry Eye Questionnaire and McMonnies questionnaire.7-13 While others are available, these have been validated, standardized and shown to assess multiple dimensions of frequency and severity of symptoms.14-19 Studies show that symptoms correlate poorly with signs.19,20 Their true value is in being able to establish “soft” benchmarks to gauge the effectiveness of your management plan in relieving symptoms.
2. Tear film/lens interactions. Research has established that contact lenses disrupt the normal dynamics of the tear film and reduce its thickness.21,22 This can contribute to discomfort and intolerance.23-27 For this reason, it is important to assess the quantity, quality and interactions of the tears with the surface of the contact lens (Figures 1a and 1b). This is done during the course of your biomicroscopic examination of the patient. Patients at risk will often show an abundance of debris in the tear film, a reduced tear prism height, and a rapid thinning or evaporation of the pre-lens tear film (Figure 2).21,28,29
Sophisticated tear film measuring devices making their way out of research labs and into our clinics include interferometers and tomographers.2,30,31 Two more common devices are the Keratograph 5M (Oculus) and Lipiview (TearScience) units.33,34 These instruments, designed to assess tear film properties without a lens on the eye, are helpful for identifying at-risk wearers. Their value in assessing the tear film during lens wear has yet to be scientifically established. The K5M is a multifunctional tool that can provide topographical information, pupilometry, tear film analysis, meibography, oxygen maps, color imaging and contact lens fitting support (Figures 3a and 3b).
| Figure 2: Keratograph 5M assessment of tear prism height. |
3. Lens wetting. The issues associated with lens wettability and the lack of agreement on how to best measure it is complicated. Despite the lack of conclusive evidence and challenges to reliably measuring this attribute, wettability may contribute to lens intolerance and discomfort.35 The surface and bulk properties of the polymers used in the manufacture of contact lenses are manipulated by chemists to enhance the attraction, retention and release of moisture from a particular lens. You can subjectively evaluate the wettability of the lens clinically by assessing for an even distribution and stability of the pre-lens tear film, but recognize the limitations of interpreting the findings.
4. Meibomian gland assessments. Evidence shows contact lens wear is associated with changes in meibomian gland morphology.36-38 The relationship between symptoms of dryness and observed changes in the meibomian glands may be related more to artifacts from the testing method than an actual causal relationship.39,40 Evidence shows this may result in instability of the lipid layer, hastening evaporation of the pre-lens tear film.3 This, in turn, leads to intolerance and discomfort. Visual inspection of the lid margins is important. Expressing the lid with digital pressure against the globe or a flat metal instrument, between two applicators, or with the meibomian gland expressor, is a simple way to assess patency of the orifices and quality of the secretions.41
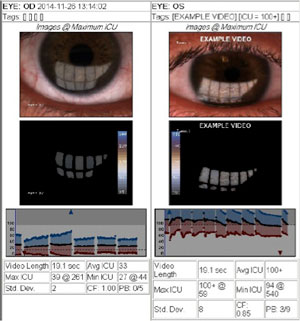 | |
| Figure 3a: Lipiview interferometry assessment of the lipid layer thickness in a patient with evaporative dry eye. | |
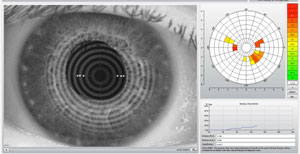 | |
| Figure 3b: Keratograph 5M assessment of non-invasive tear break-up times. |
Using a transilluminator held on the external side of an everted lid can allow you to visualize the number, shape and extent of the meibomian glands. A growing number of devices use infrared imaging to enhance the contrast between the glands and surrounding structures.42 Atrophy, drop out and tortuous dilations of the glands are all signs of dysfunction that may indirectly contribute to symptoms (Figure 4).
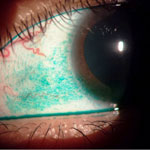
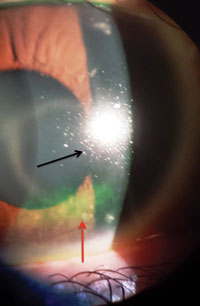
5. Corneal and conjunctival staining. If you have not been routinely using lissamine green or fluorescein as part of your soft lens patient evaluation because of fear of staining their lenses or the time it may require to irrigate the stain, you are missing out. I will leave discussions of high molecular weight fluorescein and differences in uptake between hydrogel and silicone hydrogels aside. Multiple authors have found staining to be more prevalent in contact lens wearers and associated with discomfort (Figures 5a, 5b and 5c).43-47
Corneal staining’s mechanism and its potential impact on ocular health should be part of your calculus in troubleshooting. This staining can tip you off to meibomian gland dysfunction (inferior third of the cornea), ocular surface disease (in any region), solution-induced staining (typically diffuse) and mechanical lens-ocular surface interactions (isolated regions).2 Document your findings consistently using any number of standardized scales.48 Pay particular attention to markers of dryness in the lid-wiper region of the upper lid after staining with lissamine green. Prevalence rates of staining are at least two and a half times higher in symptomatic contact lens wearers than asymptomatic wearers.49,50 Lid parallel conjunctival folds (LIPCOFs) may also represent damage from the mechanical friction between dry surfaces and have been shown to be associated with lid wiper epitheliopathy (LWE) and dry eye in contact lens wearers (Figures 6 and 7).51
Systematic Management
Once you have identified the root cause for the patient’s intolerance and reduced wearing time, it’s time to strategize a treatment. Since it is a multifactorial problem, the solution frequently requires a multifaceted approach. I prefer an approach that provides both short-term relief of symptoms and a long-term attack on the underlying issues. This demonstrates empathy for patients by addressing their acute complaints, engages their compliance and buys time to address any chronic physiological issues.
 | |
| Figure 4: Keratograph 5M assessment of deterioration of meibomian gland morphology as evidenced by atrophy, attenuation, tortuosity and dilation of acinar lumen. |
Here are the recommendations from the International Workshop on Contact Lens Discomfort based upon the review of the scientific literature (Table 2).2 Let’s take them one at a time in an attempt to develop a methodical approach. An underlying assumption is that you have perfected the lens fit in accordance with the manufacturer fitting guides and your clinical judgment and have addressed any coexisting factors such as autoimmune or atopic disease, including lid or tear film disease, structural issues with lid anatomy and corneal or conjunctival diseases.3
1. Changing replacement interval. The thought behind increasing replacement frequency centers on the belief that lens soilage leads to discomfort. It is nearly impossible to separate out replacement frequency from other factors contributing to contact lens comfort.52 Many studies looked at hydrogel lens wearers who converted from conventional to either quarterly or monthly replacement intervals.2 Given the preeminence of silicone hydrogels and the decline of both conventional and quarterly replacement, these findings may not be so useful. The evidence for increasing replacement intervals from monthly to biweekly, resulting in improved comfort, remains equivocal.2 But, research shows improved comfort for patients who switch to daily disposables.53 The bigger issue, regardless of frequency of replacement, appears to be patients exceeding manufacturer recommended replacement intervals.54
 |  | |
 | ||
| Figure 5a (top left): Dense area of lissamine green staining in a case of severe dry eye with underlying meibomian gland dysfunction. Figure 5b (right): Black arrow indicates debris and unstable tear film after expression of the meibomian glands. Red arrow indicates a dense area of inferior fluorescein staining associated with meibomian gland dysfunction. Figure 5c (bottom left): Fluorescein staining of a second patient with meibomian gland dysfunction. | ||
2. Changing materials. Recognize that, when changing materials, you are also changing many other parameters such as the surface or bulk properties, FDA group and edge profiles or thickness. Methodological study differences further complicate arrival at a consensus on the perfect material. Each material offers a rational basis for its existence, though it is clear there are differences among them. It is difficult, under these circumstances, to know why a change produces a certain result, making it challenging to reproduce the positive effects for an individual patient. Nonetheless, there are multiple studies demonstrating improvement in symptoms of dryness when hydrogel wearers are converted to silicone hydrogels.55-60 There is continuing debate among stakeholders regarding the separation of silicone hydrogels into multiple new FDA groups, which may better reflect material differences within this category of lenses.
3. Care systems. Those of us who began fitting contact lenses when the dinosaurs roamed the earth can share stories of thimerosal and chlorhexidine hypersensitivities. There is nothing new about lens solution interactions, but practitioner awareness was piqued by the Fusarium and Acanthamoeba events of 2006 to 2008. This has stimulated investigators to dive into the issues with renewed vigor. Invariably, discussions include comfort issues as well as concerns over compliance and adverse events. This is an important point. Comfort and adverse events for a given lens/solution combination do not necessarily go hand in hand, requiring each be considered separately. The important takeaway regarding comfort is that changing solutions to improve comfort may be effective but confusing.61,62 Peroxide systems are currently enjoying market growth for their efficacy and low rates of adverse events. This is not a useful option for intermittent wearers who may leave lenses soaking in a neutralized solution for days or longer.
| Table 2: Management approaches and strategies most helpful in ameliorating contact lens discomfort associated with dry eye. | ||
| Approach | Strategy | |
| Change care system | Switch from multipurpose and generics to peroxide-based system | |
| Eliminate care system | Switch to a daily lens product, if available in desired parameters | |
| Replacement frequency | Increasing replacement interval | |
| Change lens design or material | Move to lower water content hydrogels or silicone hydrogels | |
| Tear supplementation | Consistent use of lubricants, wetting agents, inserts and/or punctal occlusion | |
| Dietary supplementation | Omega-3s and 6s (e.g. primrose, flaxseed, fish or krill oils) | |
| Topical medication | Azithromycin 1%; cyclosporin A 0.05% and steroids are of little use | |
| Improve environment | Computer vision syndrome, visual breaks, reduce drafts and dust | |
A seemingly simple approach is to eliminate care systems altogether by going to daily replacement, but as discussed above this is not always a panacea. Not only do the surface and bulk properties as well as edge profiles need to be considered, but the packaging solution may also contain wetting agents that may be irritating to sensitive patients. Keep this in mind, particularly if discomfort develops early in the wear cycle or upon insertion, and consider having the patient rinse the lens with saline before insertion.
4. Tear supplements/wetting agents. The use of tear supplements, rewetting agents, lubricants and artificial tears can be useful in providing symptomatic relief for contact lens wearers experiencing dry eye, research shows.2 In some cases it may be placebo effect rather than actual changes to the surface properties of the eye and contact lens. Attributes of topical agents deemed to be beneficial include hypo-osmotic status; non-preserved formulation or use of 2% povidone preservative; and presence of carboxymethylcellulose (CMC) or polyvinyl alcohol.2
Older technologies shown to provide relief are sometimes forgotten, but are still available, such as hydroxypropylcellulose inserts.63,64 Don’t forget that punctal plugs have utility here, but plug both upper and lower lids for best effect.2 Both technologies are indicated for the management of symptomatic moderate to severe dry eye, keratoconjunctivitis sicca and may be viewed as additive to other coexisting therapies.1
5. Dietary supplements. Another area where substantive evidence exists for how to improve dry eye symptoms is the use of essential free fatty acids, specifically omega-3s and omega-6s. The DEWS and MGD workshops cover their anti-inflammatory efficacy in great detail.1,3 Evening primrose oil has been evaluated in a group of soft lens wearers, demonstrating improvements in comfort and tear prism heights.65 Other supplements such as fish, krill and flaxseed oils may produce similar benefits.1 The strategy is to manage the underlying dry eye problems to improve the ocular surface environment and reduce evaporative tear loss.
6. Topical medications. Some may consider the use of topical agents with anti-inflammatory properties a temporary measure for managing acute inflammation since their long-term safety has not been established in contact lens wearers. Concerns include toxicity from the binding of preservatives to reusable lenses, development of antibiotic resistance, opportunistic infection and risks of cataract or glaucoma in the case of steroids. The most commonly advocated agents for which there is evidence of efficacy are azythromycin 1% solution, cyclosporin A 0.05% emulsion and steroids or NSAIDs.2,66-69
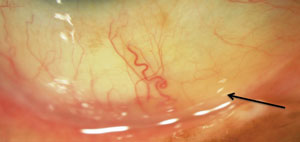 | |
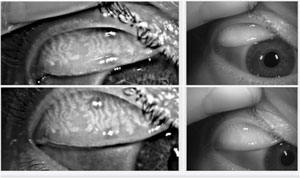
| Figure 6: Arrow indicates lid parallel conjunctival folds (LIPCOF). | |
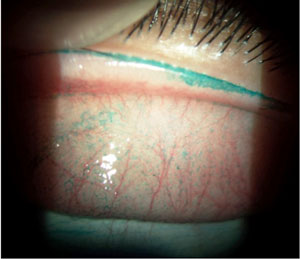 | |
| Figure 7: Lissamine green staining demonstrating lid wiper epitheliopathy (LWE). |
Steroid and NSAID use has not been studied in sufficient detail to assess their merits beyond acute relief of symptoms.
It is clear more rigorous studies are needed to identify the modifiable factors contributing to dry eye symptoms in contact lens discomfort, which tests are most diagnostic and which management strategies are most effective. Nonetheless, searching for underlying ocular surface pathologies, symptoms and signs is a jumping off point. Treating the underlying problems in conjunction with a methodical (often multi-pronged) treatment approach can allow wearers to comfortably increase their wearing times or continue to wear contact lenses. Dry eye issues need not result in patients dropping out of contact lens use altogether in most cases. Apply the scientific literature critically to each individual’s needs and improve the quality of your patient’s lives.
Dr. Fuller is an associate professor and founding supervisor of the cornea contact lens refractive surgery residency at The Eye Center, Southern College of Optometry.
1. Foulks GN. 2007 Report of the international dry eye workshop (DEWS). Ocular Surf 2007;5(2):81-6.2. Nichols JJ, Jones L, Nelson JD, et. al. The TFOS Workshop on contact lens discomfort. Invest Ophthalmol Vis Sci 2013;54(11):1-156.
3. Nichols KK, et. al. The International Workshop on Meibomian Gland Dysfunction. Invest Ophthalmol Vis Sci 2011;52(4):1917-2085.
4. Rohit A, Willcox M, Stapleton F. Tear lipid layer and contact lens comfort: a review. Eye Contact Lens 2013. May;39(3):247-53.
5. Rohit A, Willcox MD, Brown SH, et al. Clinical and biochemical tear lipid parameters in contact lens wearers. Optom Vis Sci. 2014 Dec;91(12):1384-90.
6. Sullivan B, Crews L, Messmer E, et al. Correlations between commonly used objective signs and symptoms for the diagnosis of dry eye disease: clinical implications. Acta Ophthalmol. 2014 Mar;92(2):161-6.
7. Walt J, Rowe M, Stern K. Evaluating the functional impact of dry eye: the Ocular Surface Disease Index (Abstract). Drug Inf J:1997;31:1436.
8. Dougherty BE, Nichols JJ, Nichols KK. Rasch analysis of the Ocular Surface Disease Index (OSDI). Invest Ophthalmol Vis Sci 2011 Nov 7;52(12):8630-5.
9. Chalmers RL, Begley CG, Moody K, Hickson-Curran SB. Contact Lens Dry Eye Questionnaire-8 (CLDEQ-8) and opinion of contact lens performance. Optom Vis Sci 2012 Oct;89(10):1435-42.
10. Nichols JJ, Mitchell GL, Nichols KK, et al. The performance of the contact lens dry eye questionnaire as a screening survey for contact lens-related dry eye. Cornea. 2002 Jul;21(5):469-75.
11. MCMonnies CW, Ho A. Patient history in screening for dry eye conditions. J Am Optom Assoc. 1987;58(4):296-301.
12. McMonnies CW. Key questions in a dry eye history. J Am Optom Assoc. 1986 Jul;57(7):512-7.
13. Bhatnagar KR, Pote S, Pujari S, Deka D. Validity of subjective assessment as screening tool for dry eye disease and its association with clinical tests. Int J Ophthalmol. 2015;8(1):174-81.
14. Gothwal VK, Pesudovs K, Wright TA, McMonnies CW. McMonnies questionnaire: enhancing screening for dry eye syndromes with Rasch analysis. Invest Optomthalmol Vis Sci. 2010;51(3):1401-7.
15. Khadka J, McAlinden C, Pesudovs K. Quality assessment f ophthalmic questionnaires: review and recommendations. Optom Vis Sci. 2013;90(8):720-44.
16. Ngo W, Situ P, Keir N, et al. Psychometric properties and validation of the Standard Patient Evaluation of Eye Dryness questionnaire. Cornea. 2013;32(9):1204-10.
17. Michel M, Sickenberger W, Pult H. The effectiveness of questionnaires in the determination of Contact Lens Induced Dry Eye. Ophthalmic Physiol Opt. 2009;29(5):479-86.
18. Simpson TL, Situ P, Jones LW, Fonn D. Dry eye symptoms assessed by four questionnaires. Optom Vis Sci. 2008;85(8):692-9.
19. Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea. 2004;23(8):762-770.
20. Young G, Chalmers R, Napier L, et al. Soft contact lens-related dryness without clinical signs. Optom Vis Sci. 2012;89(8):1125-1132.
21. Guillon M, Styles E, Guillon JP, Maissa C. Preocular tear film characteristics of nonwearers and soft contact lens wearers. Optom Vis Sci. 1997;74(5):273-279.
22. Faber E, Golding TR, Lowe R, Brennan NA. Effect of hydrogel lens wear on tear film stability. Optom Vis Sci. 1991;68(5):380-384.
23. Fonn D, Situ P, Simpson T. Hydrogel lens dehydration and subjective comfort and dryness ratings in symptomatic and asymptomatic contact lens wearers. Optom Vis Sci. 1999;76(10):700-704.
24. Santodomingo-Rubido J, Wolfsohn JS, Gilmartin B. Changes in ocular physiology, tear film characteristics, and symptomatology with 18 months silicone hydrogel contact lens wear. Optom Vis Sci. 2006(2);83:73-81.
25. Fonn D, Dumbleton K. Dyness and discomfort with silicone hydrogel contact lenses. Eye Contact Lens. 2003;29(1 Suppl):S101-S104; discussion S115-S118, S192-S194.
26. Glasson MJ, Hseuh S, Wilcox MD. Preliminary tear film measurements of tolerant and non-tolerant contact lens wearers. Clin Exp Optom. 1999(5);82:177-181.
27. Hom MM. Bruce AS. Prelens tear stability: relationship to symptoms of dryness. Optometry 2009;80(4):181-184.
28. Glasson MJ, Stapleton F, Keay L, et al. Differences in clinical parameters and tear film of tolerant and intolerant contact lens wearers. Invest Ophthalmol Vis Sci. 2003;44(12):5117-24.
29. Nichols JJ, Sinnott LT. Tear film contact lens, and patient-related factors associated with contact lens-related dry eye. Invest Ophthalmol Vis Sci. 2006;47(4):1319-28.
30. Finis D, Pischel N, Borrelli M, Schrader S, Geerling G. Factors influencing the measurement of tear film lipid layer thickness with interferometry. Klin Monbl Augenheilkd. 2014 Jun;231(6):603-10.
31. Huang J, Yuan Q, Zhang B, et al. Measurement of a multi-layered tear film phantom using optical coherence tomography and statistical decision theory. Biomed Opt Express. 2014;24;5(12):4374-86.
32. Lan, W, Lin, L, Yang, et al. Automatic Noninvasive Tear Breakup Time (TBUT) and Conventional Fluorescent TBUT. Optom Vis Sci. 2014;91(12):1412-1418.
33. Finis D, Pischel N, Schrader S, Geerling G. Evaluation of lipid layer thickness measurement of the tear film as a diagnostic tool for Meibomian gland dysfunction. Cornea. 2013 Dec;32(12):1549-53.
34. Keir N, Jones L. Wettability and silicone hydrogel lenses: a review. Eye Contact Lens. 2013;39(1):100-8.
35. Korb DR, Henriquez AS. Meibomian gland dysfunction and contact lens intolerance. J Am Optom Assoc. 198;51(3):243-51.
36. Henriquez AS, Korb DR. Meibomian glands and contact lens wear. Br J Ophthalmol. 1981;65(2):108-111.
37. Arita R, Itoh K, Inoue K, et al. Contact lens wear is associated with decrease of meibomian glands. Ophthalmology. 2009;116(3):379-84.
38. Paugh JJ, Knapp LL, Martinson JR, et al. Meibomian therapy in problematic contact lens wear. Optom Vis Sci. 2009;116(11):379-84.
39. Nichols JJ, Sinnott LT. Tear film, contact lens, and patient-related factors associated with contact lens–related dry eye. Invest Ophthalmol Vis Sci 2006;46(4):1319-28.
40. Korb DR, Blackie CA. Meibomian gland diagnostic expressibility: correlation with dry eye symptoms and gland location. Cornea. 2008;28(10):1142-47.
41. Pult H. Meibography in clinical practice. Ophthalmology Times 2012; (6) Available at: http://ophthalmologytimes.modernmedicine.com/news/meibography-clinical-practice?page=full. (Last accessed 5/7/2015).
42. Brautaset RL, Nilsson M, Leac N, et al. Corneal and conjunctival epithelial staining in hydrogel contact lens wearers. Eye Contact Lens 2008;34(6):312-16.
43. Maldonado-Codina C, Morgan PB, Schnider CM, Efron N. Short-term physiologic response in neophyte subjects fitted with hydrogel and silicone hydrogel contact lenses. Optom Vis Sci. 2004;81(12):911–21.
44. Lakkis C, Brennan NA. Bulbar conjunctival fluorescein staining in hydrogel contact lens wearers. CLAO J. 1996;22(3):189–94.
45. Morgan PB, Chamberlain P, Moody K, Maldonado-Codina C. Ocular physiology and comfort in neophyte subjects fitted with daily disposable silicone hydrogel contact lenses. Cont Lens Anterior Eye. 2013;36(3):118–25.
46. Guillon M, Maissa C. Bulbar conjunctival staining in contact lens wearers and non lens wearers and its association with symptomatology. Cont Lens Anterior Eye. 2005;28(2):67–73.
47. Wolffsohn JS, Naroo SA, Christie C, et al. Anterior eye health recording. Cont Lens Anterior Eye. 2015 Mar 23. pii: S1367-0484(15)00039-9.[Epub ahead of print]
48. Korb DR, Greiner JV, Herman JP, et al. Lid-wiper epitheliopathy and dry-eye symptoms in contact lens wearers. CLAO J. 2002;28(4):211-6.
49. Yeniad B, Beginoglu M, Bilgin LK. Lid-wiper epitheliopathy in contact lens users and patients with dry eye. Eye Contact Lens. 2010;36(3):140-3.
50. Pult H, Purslow C, Berry M, Murphy PJ. Clinical tests for successful contact lens wear: relationship and predictive potential. Optom Vis Sci. 2008;85(10):E924-E929.
51. Dumbleton K, Woods C, Jones L, Richter D, Fonn D. Comfort and vision with silicone hydrogel lenses: effect of compliance. Optom Vis Sci. 2010 Jun;87(6):421-5.
52. Lazon de la Jara P, Papas E, Diec J, et al. Effect of lens care systems on the clinical performance of a contact lens. Optom Vis Sci. 2013 Apr;90(4):344-50.
53. Ramamoorthy P, Nichols JJ. Compliance factors associated with contact lens-related dry eye. Eye Contact Lens. 2014 Jan;40(1):17-22.
54. Aakre BM, Ystenaes AE, Doughty MJ, et al. 6-month follow-up of successful refits from daily disposable soft contact lenses to continuous wear of high-Dk silicone-hydrogel lenses. Ophthalmic Physiol Opt. 2004;24(2):130–41.
55. Chalmers R, Long B, Dillehay S, Begley C. Improving contact lens related dryness symptoms with silicone hydrogel lenses.Optom Vis Sci. 2008;85(8):778–84.
56. Chalmers R, Dillehay S, Long B, et al. Impact of previous extended and daily wear schedules on signs and symptoms with high Dk lotrafilcon A lenses. Optom Vis Sci. 2005;82(6):549–554.
57. Schafer J, Mitchell GL, Chalmers RL, et al. The stability of dryness symptoms after refitting with silicone hydrogel contact lenses over 3 years. Eye Contact Lens. 2007;33(5):247–252.
58. Fonn D, Dumbleton K. Dryness and discomfort with silicone hydrogel contact lenses. Eye Contact Lens. 2003;29 (1 Suppl):S101–S104.
59. Young G, Veys J, Pritchard N, Coleman S. A multi-centre study of lapsed contact lens wearers. Ophthalmic Physiol Opt. 2002;22(6):516–527.
60. Diec J, Papas EB, Naduvilath T, et al. Subjective comfort and adverse events during daily contact lens wear. Optom Vis Sci. 2013;90(7):674–81.
61. Tilia D, Lazon de la Jara P, Peng N, et al. Effect of lens and solution choice on the comfort of contact lens wearers. Optom Vis Sci. 2013;90(5):411–8.
62. Luchs JI, Nelinson DS, Macy JI, Group LACS. Efficacy of hydroxypropyl cellulose ophthalmic inserts (LACRISERT) in subsets of patients with dry eye syndrome: findings from a patient registry. Cornea. 2010;29(12):1417–27.
63. McDonald M, D’Aversa G, Perry HD, et al. Hydroxypropyl cellulose ophthalmic inserts (lacrisert) reduce the signs and symptoms of dry eye syndrome and improve patient quality of life. Trans Am Ophthalmol Soc. 2009;107(12):214–21.
64. Kokke KH, Morris JA, Lawrenson JG. Oral omega-6 essential fatty acid treatment in contact lens associated dry eye. Cont Lens Anterior Eye. 2008;31(3):141–6.
65. Nichols JJ, Bickle KM, Zink RC, et al. Safety and efficacy of topical azithromycin ophthalmic solution 1.0% in the treatment of contact lens-related dry eye. Eye Contact Lens. 2012 Mar;38(2):73-9.
66. Egorova GB, Mitichkina TS, Fedorov AA, Shamsudinova AR. Topical cyclosporine for the treatment of ocular surface changes in contact lens wearers. Vestn Oftalmol. 2015 Jan-Feb;131(1):36-42.
67. Hom MM. Use of cyclosporine 0.05% ophthalmic emulsion for contact lens-intolerant patients. Eye Contact Lens. 2006;32(2):109–11.
68. Willen CM, McGwin G, Liu B, et al. Efficacy of cyclosporine 0.05% ophthalmic emulsion in contact lens wearers with dry eyes. Eye Contact Lens. 2008;34(1):43–5.

