What's Powering the Next Wave?In the September issue—our 45th annual technology report—find out how AI, telehealth, OCT and other new clinical devices are helping ODs build out their practice for the future. Check out the other featured articles here:
|
The principles of optical coherence tomography (OCT) were first described just over 30 years ago and the first commercial device came on the market in 1996. This technology offers a noninvasive approach of imaging ocular tissues with high resolution by measuring back-scattered light and producing a cross-section topographic image. With the increase in clinical implementation to manage ocular and systemic disease, optometrists must continue learning how to expand the technology’s uses from conventional and common to sophisticated but practical. These now-multimodal imaging devices can help aid in the diagnosis of both ocular and systemic disease.
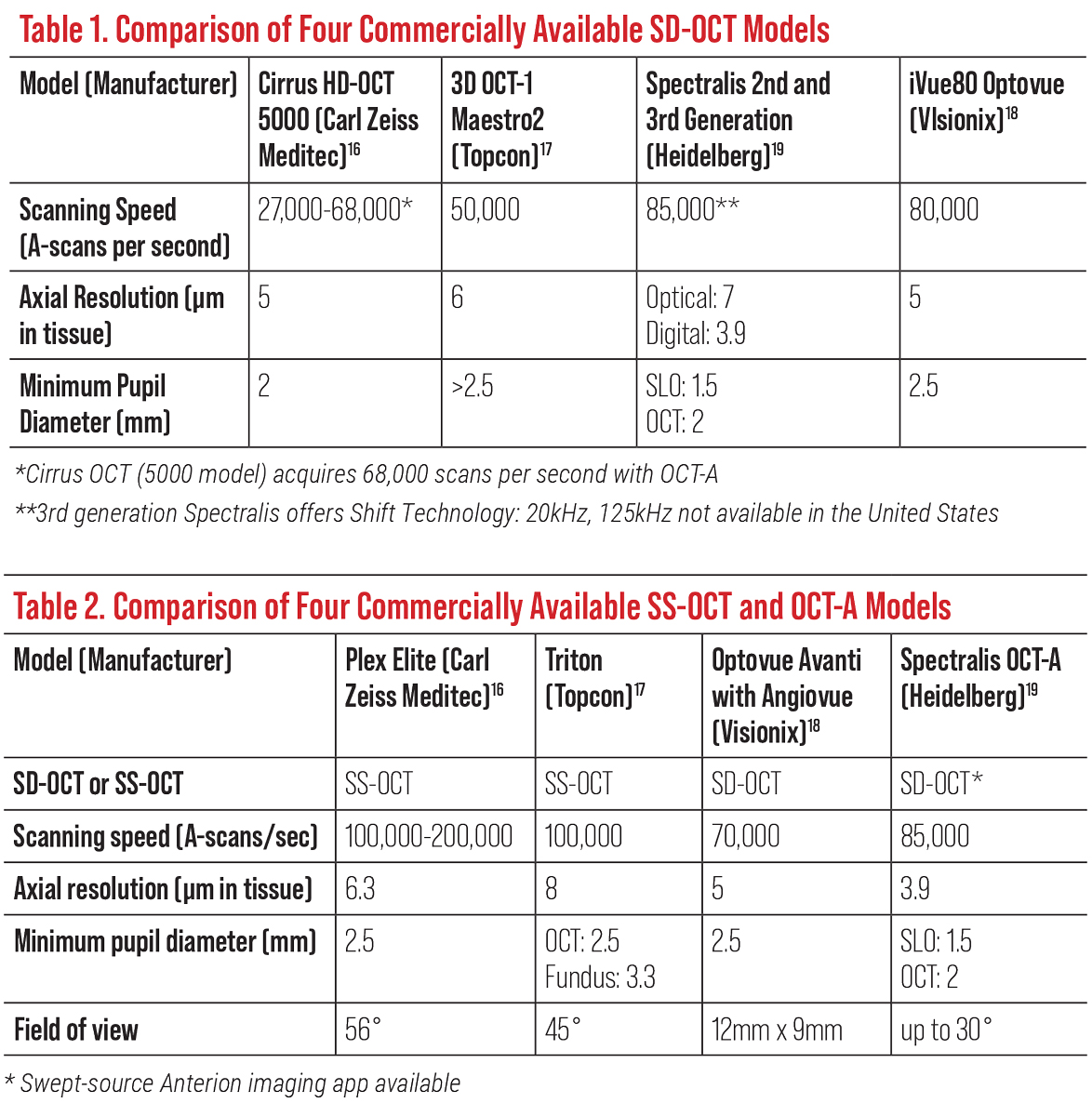
Clinicians have several commercially available spectral-domain (SD) OCT models to choose from. For this article, four prominent ones will be described: Cirrus HD SD-OCT 5000 (Carl Zeiss Meditec), 3D OCT-1 Maestro2 (Topcon Healthcare), Spectralis SD-OCT (Heidelberg Engineering) and Optovue iVue80 (Visionix). This article outlines the basics of OCT imaging, the newest model updates, a guided data analysis of the different instruments’ metrics and how to interpret various measurements using each model.
 |
| Click image to enlarge. |
OCT Basics: Then and Now
Since its 1991 debut, OCT technology has continually evolved. Now considered primitive, the original time-domain analysis used an infrared light source and was dependent on movement of a mirror to change the optical path of a reference beam. It could only acquire 400 scans per second with an 840nm wavelength, which limited imaging resolution and sampling density.2
Current spectral-domain OCT (SD-OCT) offers a superior resolution with a lessened acquisition time and can capture between 26,000 and 80,000 axial scans per second, while swept-source OCT (SS-OCT) can achieve upwards of 100,000 to 200,000 axial scans per second (Tables 1 and 2).3 This development allows the clinician to minimize motion artifacts and provides volumetric analysis with three-dimensional imaging capabilities to obtain better quality images.2
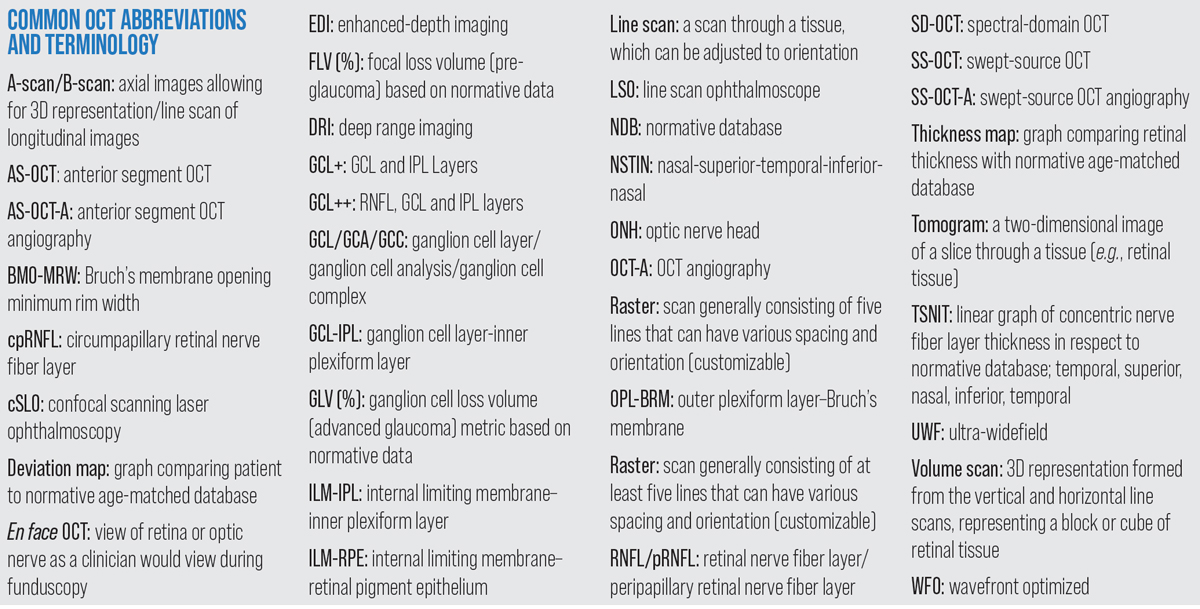 |
| Common OCT abbreviations and terminology. Click image to enlarge. |
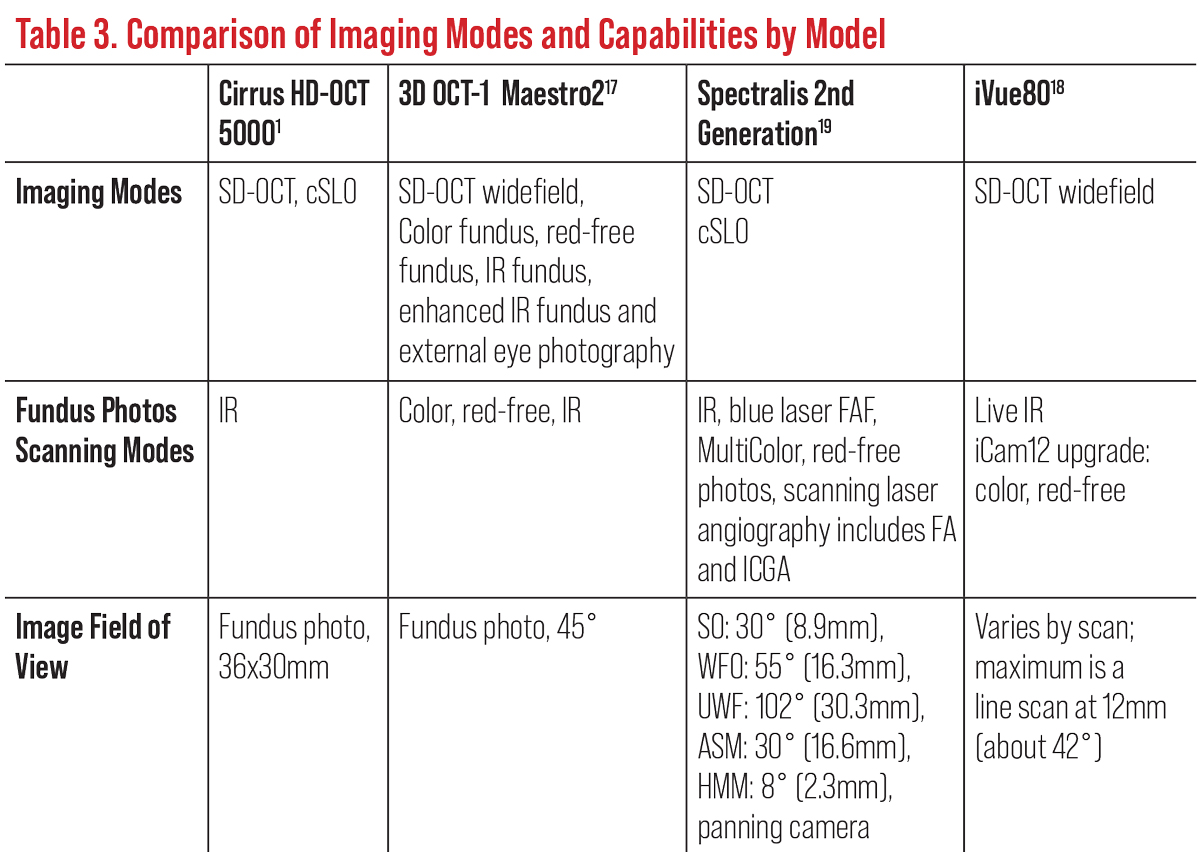
Today, the most common OCT models on the market for eye care are the combination OCT/fundus systems and advanced OCT models. These SD-OCT devices have enhanced fundus camera capabilities, increased field of view and can combine retinal nerve fiber layer (RNFL), macula and ganglion cell layer (GCL) all in one report (Figures A and B). Multimodal imaging offers full-color, high-resolution fundus photography, reflectance imaging and fundus autofluorescence.
Many of the newer SD-OCTs have more anterior segment options to better evaluate the cornea and anterior chamber (Figures I to L). Most continue to allow image acquisition with miotic pupils, which is advantageous for patients who struggle with dilation due to age or other factors.4 Furthermore, advancements and upgrades in OCT systems include widefield, swept-source, enhanced-depth imaging (EDI-OCT) and OCT angiography (OCT-A) technologies. These will all be discussed below.
 |
| Click image to enlarge. |
SS-OCT and EDI-OCT allow for deeper retinal imaging that includes the choroid, with faster acquisition speeds.5 SS-OCT uses longer wavelengths (1050nm vs. 840nm in SD-OCT) to overcome scattering light defects of the RPE and faster scanning times (100,000 to 400,000 A-scans/sec) allow for longer B-scans to help with widefield imaging.2,3 With these parameters, SS-OCT allows for penetrance to the level of the choroid with a reduced axial resolution.6
Two commercially available SS-OCTs today are Topcon’s Triton DRI (Figure H) and Plex Elite 9000. They offer 12x9mm widefield scans.7 EDI-OCT penetrates an additional 500µm to 800µm deeper compared with traditional OCT.1 The EDI feature has become the gold standard of detection of optic nerve head drusen and very useful in age-related choroidal atrophy, high myopia, central serous chorioretinopathy and choroidal tumors.8,9
 |
| Click image to enlarge. |
Currently, EDI scans are available on the 4000 and 5000 Zeiss models as well as the second and third generation Spectralis models. Topcon uses similar technology, called “deep range imaging” (DRI) on its systems, while Optovue offers “deep choroidal imaging” (DCI). The EDI option may have to be chosen when taking the scans on SD-OCT. SS-OCTs integrally image the choroid and deeper structures without the need for EDI.
Several of the new SD and SS models have a true color fundus photo with corresponding disc topography, RNFL thickness, GCL thickness with reference data all on the same report (Figures A and H). Spectralis has a multicolor imaging platform offered with confocal scanning laser ophthalmoscope (cSLO) fundus imaging (Table 3).
 |
| Click image to enlarge. |
Typically thought of as a posterior segment diagnostic instrument, the OCT has offered several different anterior segment options (Figures I-L). In addition to aiding in the assessment of the cornea and angle, anterior segment OCT (AS-OCT) can also be used to fit complex specialty contact lens patients. AS-OCT has the capabilities of measuring sagittal height and depth, tear layer clearance, landing zone and thickness alignment for scleral, rigid gas permeable and custom fit lenses.10
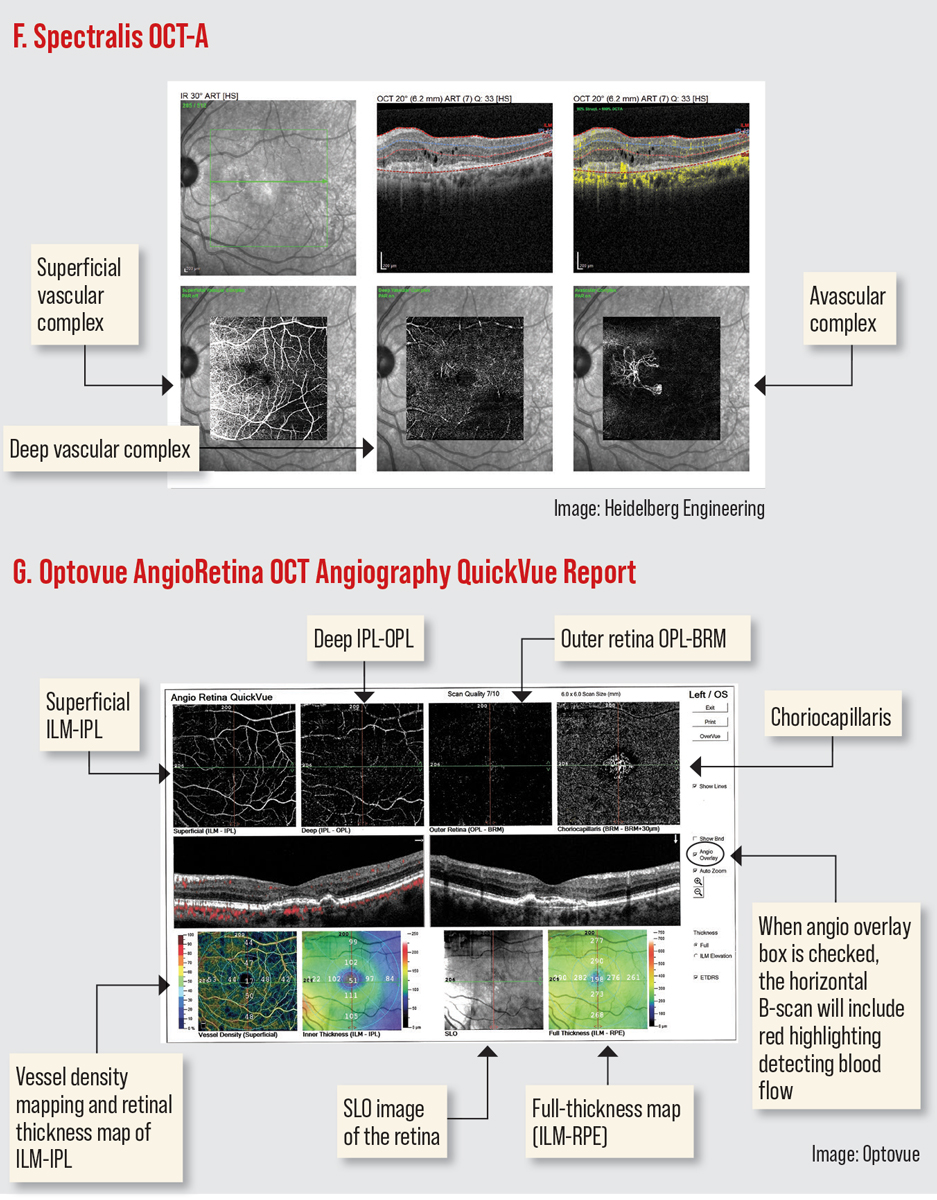
Phase-variable technology, or OCT-A, combines Doppler analysis and OCT imaging to produce superimposed structural data.4,6 Its noninvasive technology obtains three-dimensional volumetric images of the retinal and choroidal vascular systems as well as blood flow. These images are dependent on multiple cross-sectional scans, scattering properties and collecting depth-encoded profiles from motion contrast of erythrocytes.5 OCT-A has improved identification of capillary nonperfusion, microaneurysms and retinal ischemia, as well as delineating the foveal avascular zone.11
Table 2 references the four main commercially available OCT-A devices with their respective angio analytics. While montage OCT-A is useful in detecting small areas of neovascularization and capillary nonperfusion, it is not required to detect small areas of neovascularization and capillary nonperfusion. However, in diabetic retinopathy, many of these areas are going to be outside of the typical 6x6mm or 8x8mm scan; a montage strategy allows for a wider field of view to detect these lesions.1
 |
| Click image to enlarge. |
Furthermore, OCT-A imaging blood-flow information can be displayed as en face and the cross-sectional view. An en face view allows blood flow visualization as a top view mode and can separate the inner and outer retinal vascular plexus, the choriocapillaris and the choroid (Figures F and G). It can also be used to look for blood flow in regions where it should not be, such as on the vitreoretinal interface in the situation of retinal neovascularization and in the avascular zone with the development of choroidal neovascularization. Cross-sectional views offer blood flow information displayed over a structural B-scan, allowing detailed correlation of flow signals with structural OCT findings.12
OCT-A was initially developed as an application of SD-OCT but is now being integrated into SS-OCT models. Today’s instruments acquire about 70,000 to 100,000 A-scans per second using a light source centered at a wavelength of either 840nm or 1050nm, for SD- or SS-OCT-A, respectively, with an interscan time of 4ms to 5ms, and achieving optical axial resolution of 5µm to 10µm.5 Blood flow velocity in the retinal circulation can be anywhere in the range 0.2mm/s to 7mm/s.5 Instruments usually collect between two to four B-scans at the same location. OCT-A’s angular field of view covers 10º to 30º, but widefield can extend the raster scanning protocol to achieve much larger fields of view, up to 12×12mm using the montage technique.5,11
OCT and OCT-A have the potential to detect optic neuropathies, retrochiasmal visual pathway disorders and systemic diseases.6 In particular, SD-OCT has revealed diffuse inner retinal thinning that is prominent of the RNFL, GCL/inner plexiform layer (IPL) junction and choroid. These are commonly seen in patients that suffer from neurodegenerative diseases such as Alzheimer’s, multiple sclerosis and dementia. (Figure M).13,14 Alzheimer’s disease patients showed a significant thickness reduction in global and temporal superior quadrants in RNFL.15 OCT-A analysis found a reduction of retinal vessel and perfusion density, with a larger foveal avascular zone.13
 |
| Click image to enlarge. |
Comparing OCT Scan Reports
While all four SD-OCT models are comparable in many ways, each one displays the data differently.
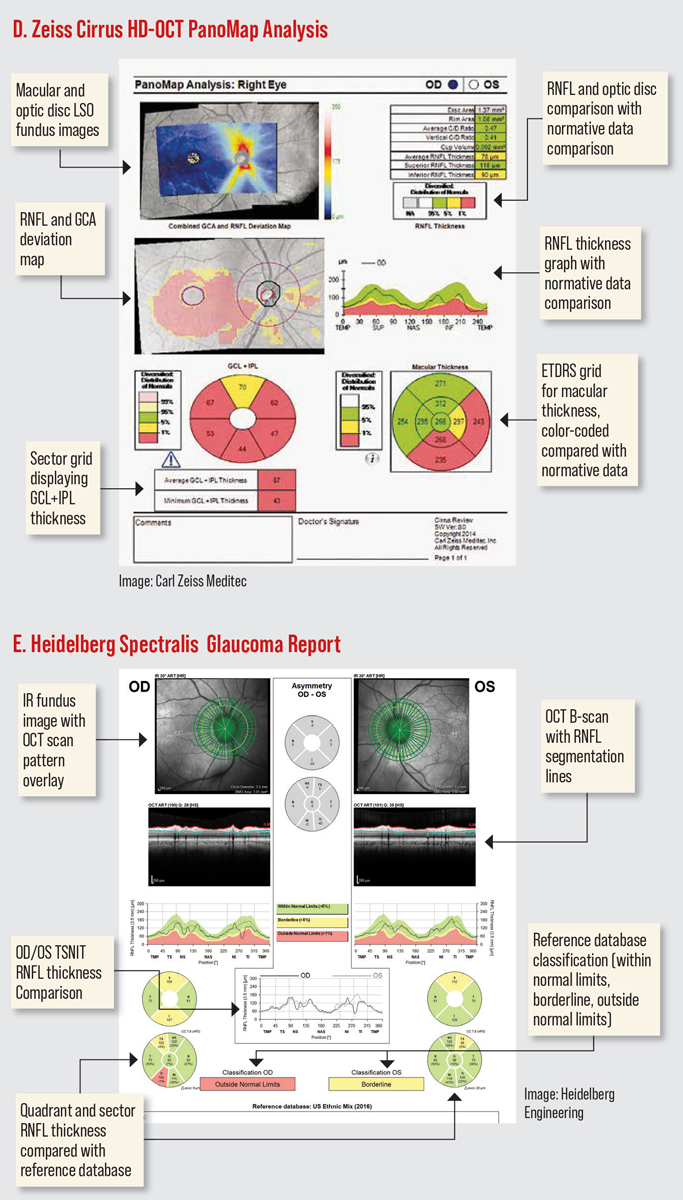
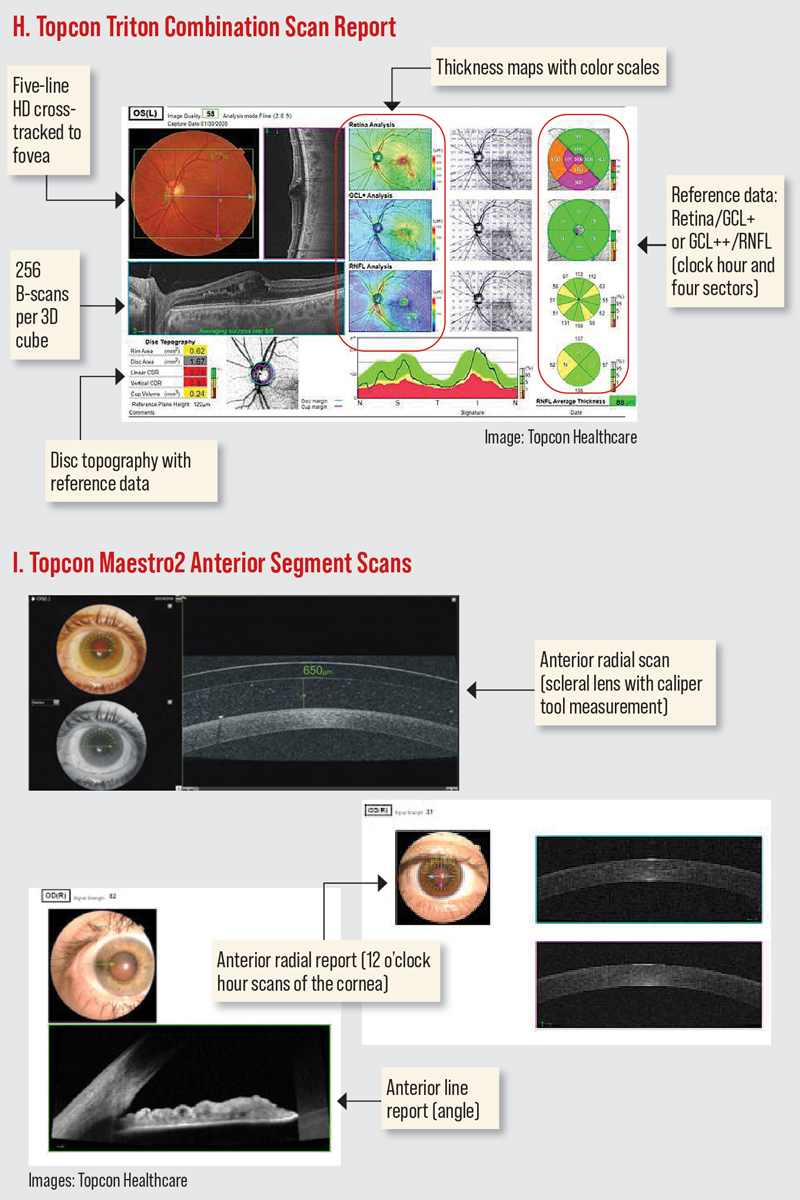
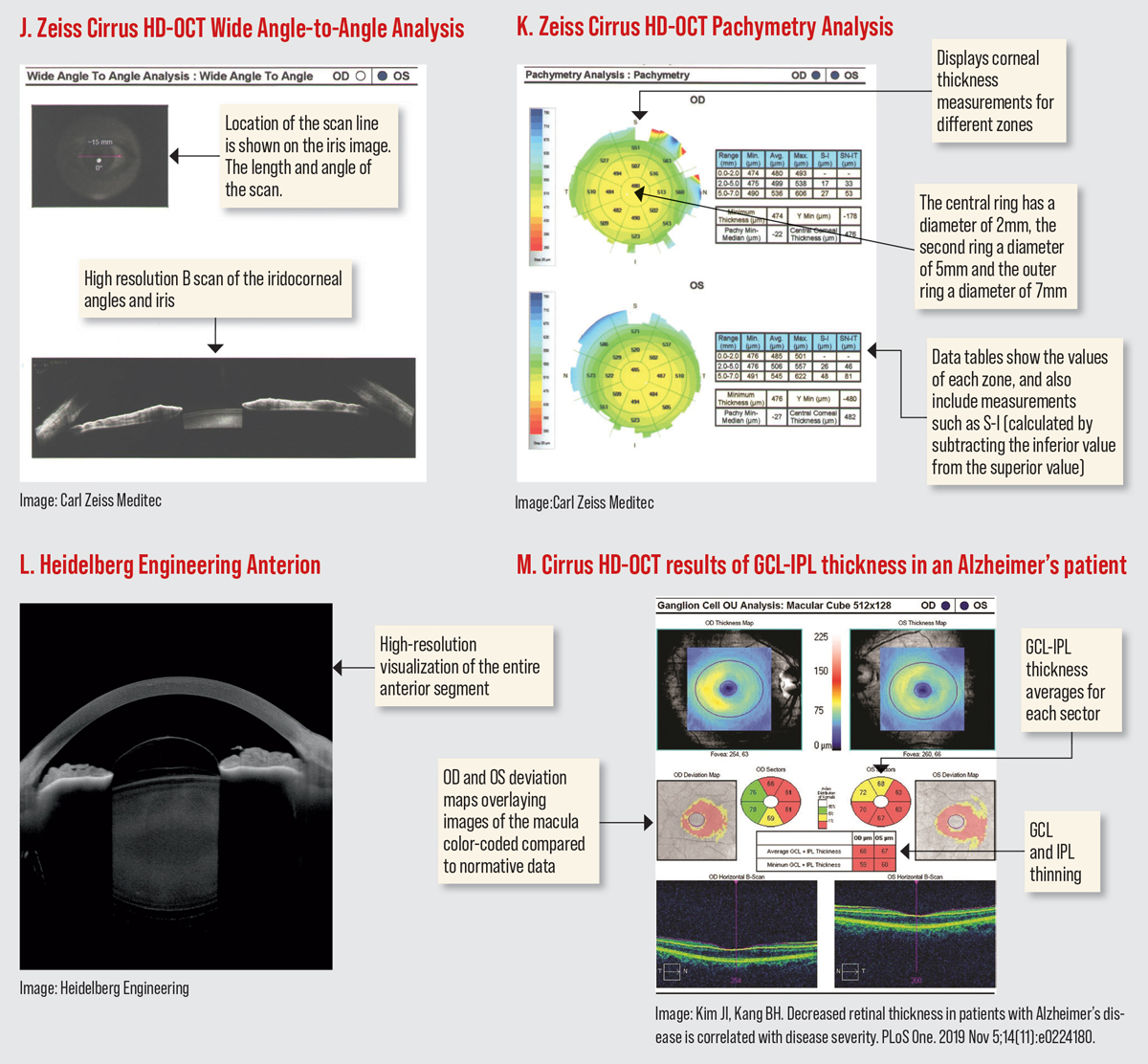
The Cirrus HD-OCT has a PanoMap Analysis report that combines information from the macular cube and optic disc cube scans, providing an integrated widefield viewpoint for comprehensive analysis which is particularly useful in monitoring glaucoma progression. (Figure D). The newest software has several anterior segment scan options. The HD angle scan generates a report that is used for the assessment and documentation of the anterior chamber angle. The pachymetry scan uses 24 radial scan lines to generate a color-coded map of the cornea. The Wide Angle-to Angle scan captures both iridocorneal angles in one scan. (Figures I-J).16
The 3D OCT-1 Maestro2 offers widefield OCT with color retinal photography. In addition to automated capture, the Maestro2 offers manual/semi-manual options for difficult-to-image patients.17 The 3D wide report offers simultaneous imaging of the macula and optic nerve in one scan, providing a single report with retinal thickness, RNFL, ganglion cell thickness and optic nerve head (ONH) analysis with reference database comparisons (Figure A). The Maestro2 also has several anterior segment scan options (anterior radial and anterior line; Figure I).
 |
| Click image to enlarge. |
The iVue80 (Optovue) offers 80,000 A-scans per second, which is three times faster than the original iVue OCT. The most current model offers en face imaging. Focal loss volume (FLV%) and global loss volume (GLV%) are GCC metrics unique to this system that provide information to aid in glaucoma diagnosis and management.18 (Figure B) The retina map compares OD/OS on the same report (Figure C).
The third-generation Spectralis SD-OCT offers 85,000 A-scans per second compared to 40,000 in the first-gen device. In addition to EDI, Spectralis second and third generations offer enhanced vitreous imaging. Spectralis has something called shift technology, which the manufacturer says enables you to switch between three OCT scan speeds to find the optimal balance of image quality and clinical workflow. It received the CE Mark for safety in Europe in 2022, but is not yet available in the United States. Spectralis now offers Anterion SS-OCT imaging, which provides high resolution visualization of the entire anterior segment (Figure L).19
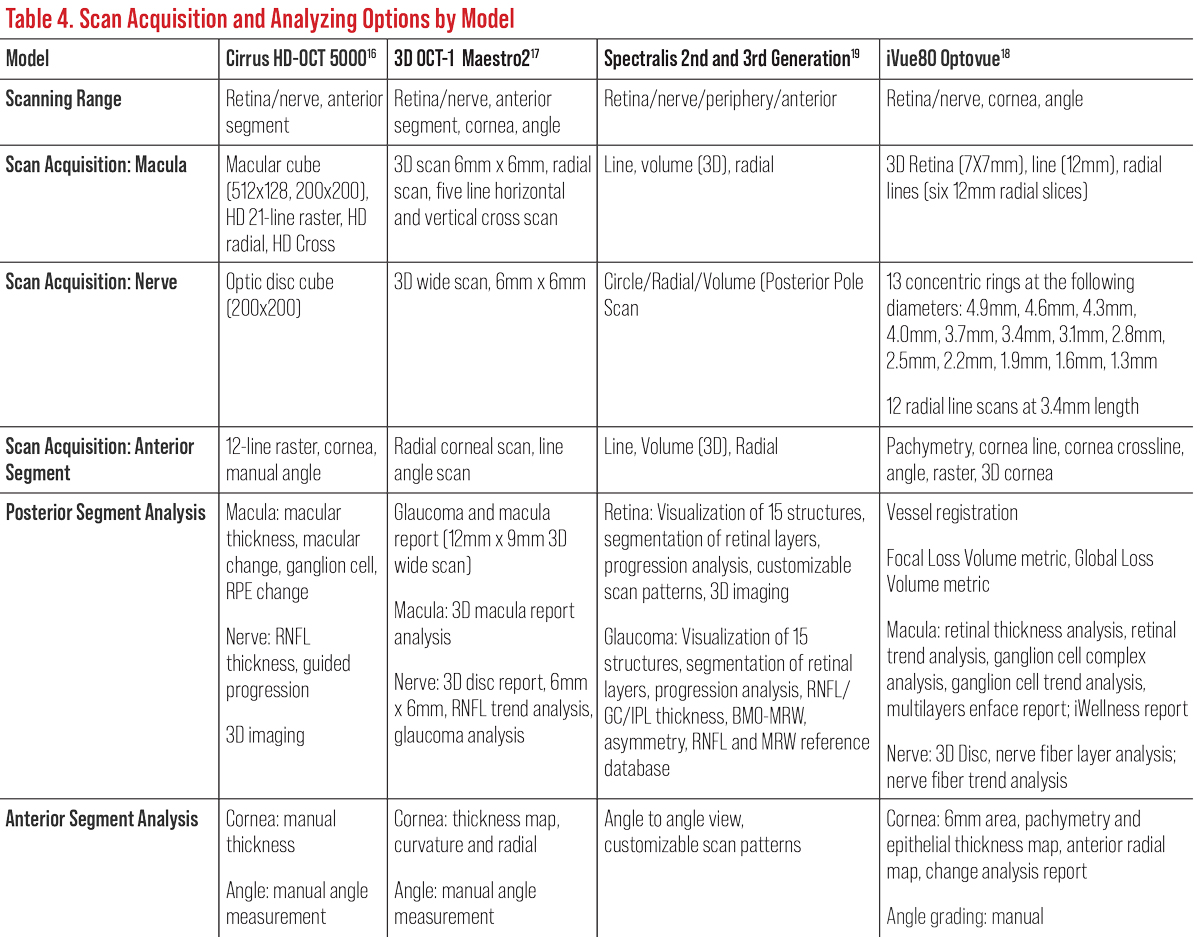
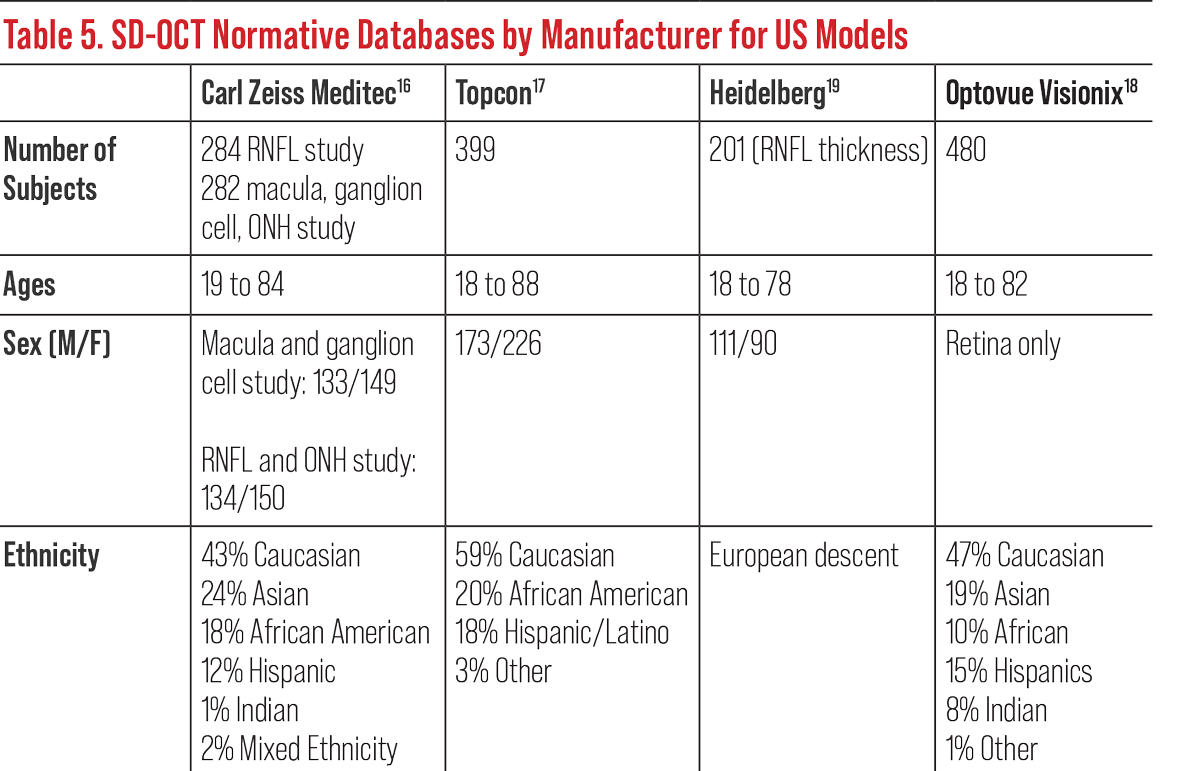
All OCT instruments possess a retinal thickness scanning mode as well as an optic nerve and RNFL acquisition and analysis. Most have an anterior segment option to evaluate the anterior chamber and cornea (Table 4). Current OCTs also possess recognition software and progression or change functions to track and monitor progression over time based on each model’s normative data (Table 5).
 |
| Click image to enlarge. |
OCT-A’s Limitations
Despite the technological wonder of better understanding retinal vasculature in a noninvasive approach, several limitations currently exist. These can be divided into technological and uniform standardization deficits. Currently, OCT-A’s image quality is impacted by artifacts produced by motion, such as blinking and saccades.20 Also, OCT-A does not currently provide dynamic information such as velocity of blood flow or the presence of leakages.21
In order to increase the visibility and orientation of retinal vessels, reduce imaging artifacts and improve post image processing, a dual-mode instrument that has standard (20µm) and high transverse resolutions (5µm to 10µm) is beneficial. Working on advancing from 2D projections to 3D will improve volumetric vascular networks and metrics, as well as increasing the angular field of view to more than 100º.
Commercially available instruments currently do not have normative databases from which to compare your individual patients. More comprehensive multi-sourced databases are needed to incorporate this into the instruments. This will aid in the diagnosis of retinal vascular disease and identify potential biomarkers for a variety of retinal and systemic pathologies. Currently, two OCT-A datasets can be found from the ROSE and PREVENT studies.5
 |
| Click image to enlarge. |
The Future of OCT
After 2020, the global market for OCT in ophthalmology was estimated at $526.2 million in the United States and is projected to reach approximately $754.4 million in the US by 2026.22 Further advancements in macular and extramacular imaging with adaptive optics are to include indocyanine green angiography and widefield OCT-A with eye motion artifact correction and laser Doppler holography. This technology permits a large field of blood flow imaging and deeper assessment of the retina and choroid anterior to the equator with high resolution.23
Additionally, swept-source systems and three-dimensional visualization of choroidal vessels are being evaluated to visualize the choroid vasculature.24 Promising early-stage lab-based research includes the study of retinal vascular function, including monitoring of pulse wave velocity, blood flow heterogeneity and response to external stimulation.5 Other widefield OCT devices that use both SD and SS-OCT technologies are under development.
Research on the anterior segment OCT-A remains in its initial phase. With an adaptor lens, anterior segment OCT-A analysis can be used in assessing the conjunctiva, cornea and limbus, ciliary body and iris. OCT-A can also scan the anterior segment for abnormal vasculatures and assess limbal epithelial stem cells and suspicious lesions. Postoperative use of anterior segment OCT-A will help in the early evaluation of blood flow density on filtering blebs after trabeculectomy. Widefield SS-OCT-A shows potential for anterior segment imaging due to its sufficient scanning range and high sensitivity in detecting structures.11
Portable, handheld OCT devices with and without angiography are being better refined to offer disease management in pediatric and elderly patients. These devices are becoming smaller with more accurate and detailed results of direct imaging, and manufacturers are creating normative databases.25 Currently, at-home OCT monitoring is being developed for exudative macular degeneration with the Notal Home OCT by Notal Vision. This device is said to be patient friendly for those with reduced acuities (>20/400) and uses artificial intelligence software to analyze for subretinal fluid. If detected, a report is generated and assigned to a physician at the Notal Vision Diagnostic Clinic for further interpretation and instructional follow-up recommendation.26
Collectively, the recent and upcoming advancements have expanded the utility of OCT, making it an important ancillary test for clinicians. With its multimodal imaging, OCT can offer more than one diagnostic image to help primary eyecare clinicians to understand more about the underlying pathogeneses, disease progression and treatment responses to further improve patient care.
Dr. Pennington is a staff optometrist at the Hampton VA Medical Center, where she is adjunct faculty for The Ohio State University College of Optometry. She is the president of the Tidewater Optometric Society and a member of the board of trustees for the Virginia Optometric Association.
Dr. Smith is a staff optometrist at the Hampton VA Medical Center, where she is the externship director and adjunct faculty for The Ohio State University. She is an active Fellow of the American Academy of Optometry where she lectures and currently sits on the fellowship committee advisory board. Neither has any financial disclosures.
1. Majcher C, Cobbina S. Take OCT to the next level. RevOptom. 2021;158(9):32-40. 2. Levison A and Ehlers J. “Widefield oct: current and future applications. Retinal Physician, April 1, 2014. www.retinalphysician.com/issues/2014/april-2014/widefield-oct-current-and-future-applications. Accessed July 15, 2022. 3. Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254(5035):1178-81. 4. Mazzarella J, Cole J. The anatomy of an OCT scan. RevOptom. 2015;152(9):58-68. 5. Sampson DM, Dubis AM, Chen FK, Zawadzki RJ, Sampson DD. Towards standardizing retinal optical coherence tomography angiography: a review. Light Sci Appl. 2022;11(1):63. 6. Lo C, Vuong LN, Micieli JA. Recent advances and future directions on the use of optical coherence tomography in neuro-ophthalmology. Taiwan J Ophthalmol. 2021;11(1):3-15. 7. Salsberg K. Swept-source and multimodal OCT technologies offer clinical advantages. Optometry Times. June 12, 2020. www.optometrytimes.com/view/swept-source-and-multimodal-oct-technologies-offer-clinical-advantages. Accessed July 15, 2022. 8. Yan Y, Liao YJ. Updates on ophthalmic imaging features of optic disc drusen, papilledema and optic disc edema. Curr Opin Neurol. 2021;34(1):108-15. 9. Yiu G, Chiu SJ, Petrou PA, et al. Relationship of central choroidal thickness with age-related macular degeneration status. Am J Ophthalmol. 2015;159(4):617-26. 10. Mickles C. What OCT can offer your specialty lens fits. RevOptom. 2020;157(3):80-6. 11. Luo M, Li Y, Zhuo Y. Advances and current clinical applications of anterior segment optical coherence tomography angiography. Front Med (Lausanne). 2021;8:721442. 12. Siggel R, Spital C, Lentzsch A, Liakopoulos S. Optical coherence tomography angiography for the detection of macular neovascularization-comparison of en face vs. cross-sectional view Eye (Lond). January 6, 2022. [Epub ahead of print]. 13. Song A, Johnson N, Ayala A, Thompson AC. Optical coherence tomography in patients with Alzheimer’s disease: what can it tell us? Eye Brain. 2021;13:1-20. 14. Kim JI, Kang BH. Decreased retinal thickness in patients with Alzheimer’s disease is correlated with disease severity. PLoS One. 2019;14(11):e0224180. 15. Cunha JP, Proença R, Dias-Santos A, et al. OCT in Alzheimer’s disease: thinning of the RNFL and superior hemiretina. Graefes Arch Clin Exp Ophthalmol. 2017;255(9):1827-35. 16. Carl Zeiss Meditec. Cirrus HD-OCT. www.zeiss.com/meditec/en_us/products. Accessed July 15, 2022. 17.Topcon Healthcare. 3D OCT-2000 spectral domain OCT. www.topconmedical.com/products/3doct2000.htm. Accessed July 15, 2022. 18. Visionix. iVue80 Optovue. www.visionix.com/us/. Accessed July 15, 2022. 19. Heidelberg Engineering H. Spectralis: Multi-modality diagnostic imaging of the eye. www.heidelbergengineering.com/us/products/spectralis-models/. Accessed July 15, 2022. 20. Rodman J. Is OCT-A right for my practice? RevOptom. 2020;157(9):50-6. 21. Ghasemi Falavarjani K, Tian JJ, Akil H, et al. Swept-source optical coherence tomography angiography of the optic disk in optic neuropathy. Retina. 2016;36 Suppl 1:S168-77. 22. PR Newswire. Global industry analysts predicts the world optical coherence tomography (oct) for ophthalmology market to reach $754.4 million by 2026. March 1, 2022. www.prnewswire.com/news-releases/global-industry-analysts-predicts-the-world-optical-coherence-tomography-oct-for-ophthalmology-market-to-reach-754-4-million-by-2026--301360428.html. Accessed July 1, 2022. 23. Puyo L, David C, Saad R, et al. Laser Doppler holography of the anterior segment for blood flow imaging, eye tracking, and transparency assessment. Biomed Opt Express. 2021;12(7):4478-95. 23. Puyo L, David C, Saad R, et al. Laser Doppler holography of the anterior segment for blood flow imaging, eye tracking, and transparency assessment. Biomed Opt Express. 2021;12(7):4478-95. 25. Monroy GL, Won J, Spillman DR, et al. Clinical translation of handheld optical coherence tomography: practical considerations and recent advancements. J Biomed Opt. 2017;22(12):1-30. 26. Notal Vision. Notal Home OCT. notalvision.com/technology/home-oct. Accessed July 15, 2022. |


