Retina on the RiseExperts offer pearls for detecting and managing numerous posterior segment conditions in the June 2023 issue of Review of Optometry, our 14th annual Retina Report. Featured articles cover everything from AMD staging, referral timing, interpreting vitreous opacities and using nutrition to promote retinal health. Check out the other articles featured in this issue:
|
The vitreous body marks the largest ocular structure, composing approximately 80% of the eye. But for its extremely large size, the vitreous receives modest attention in comparison to the more alluring retina, cornea and lens. This may be attributed to the fact that primary vitreal disease is uncommon, with many developing it early in infancy and less likely for the primary optometrist to run into. Nonetheless, the vitreous plays a significant role in retinal disease development.
Why else should we pay attention to the vitreous? Well, it is responsible for a frequently heard patient complaint—floaters. Symptoms of floaters could translate clinically into a number of vitreous opacities, most often benign, but sometimes indicating a serious condition. When encountering vitreous opacities, what are we looking at and when should we be concerned? This review is dedicated to answering those questions, starting with vitreous anatomy and imaging and delving deep into breaking down vitreous opacities and treatments.
Vitreous Composition
Understanding floaters and vitreous opacities starts with a clear understanding of the anatomy and the dynamic changes the vitreous undergoes with aging. The acellular vitreous is transparent by nature as it is mostly water, about 98%. Two main macromolecules, collagen and hyaluronan, provide structure and elasticity.
The majority of the vitreous is the core, where liquefaction occurs. Surrounding the core lies the vitreous cortex, a thin layer with an increased density of collagen fibrils. The thickness of the cortex is in the range of 100µm to 300µm, being thinnest in the central macula and absent over the optic nerve.1,2 The posterior hyaloid membrane is adherent to the internal limiting membrane of the surface retina.3 The posterior hyaloid contains type IV collagen (vs. type II in the core) as well as adhesive proteins: laminin and fibronectin.3-5 Thus, it essentially functions as the glue between the vitreous and retina. The posterior hyaloid is only appreciable once posterior vitreous detachment (PVD) has occurred.
Vitreous gel liquefaction commences as young as four years old and then steadily increases with age.6,7 Liquefaction occurs as collagen aggregates into bundles, causing development of empty pockets of liquid called lacunae. The vitreous transitions from a gelatinous composition at birth to a 50:50 ratio of gel to liquid by age 80.1,7
Examination
There are multiple ways to evaluate the vitreous body, the easiest of which is by simple slit lamp and ophthalmoscopy exam. However, this method has limitations in both the extent and fine detail of vitreous changes that can be assessed due in part to the vitreous’s transparent nature.
 |
|
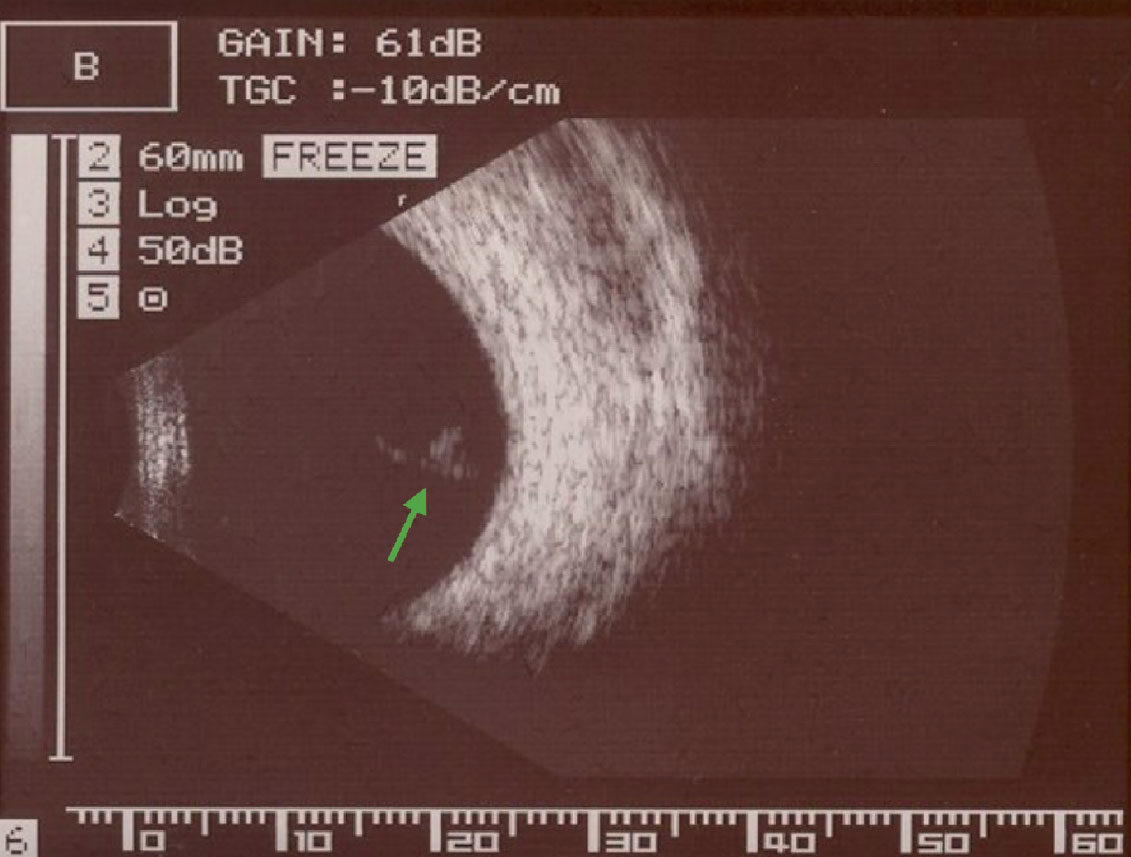
Fig. 1. B-scan ultrasonography imaging of a Weiss ring visible within the posterior vitreous after PVD. Click image to enlarge. |
B-scan ultrasound can image the entire vitreous body and can detect differences in density between liquefied and gel vitreous, as well as collagen aggregates and other opacities.8 Although not often used clinically, objective, quantitative values of the vitreous can be measured from a B-scan and have been shown to correlate with contrast sensitivity and visual function.9
OCT provides a limited view of the vitreous, specifically giving information about the posterior vitreous and vitreoretinal interface. Unfortunately, it does not image the central or anterior vitreous very well and opacities in these areas may still be responsible for symptomatic floaters. Swept-source OCT, in particular, provides better vitreous visualization over SD-OCT and allows for a wider view to image the interface across the macula and optic nerve in a single scan. Normal anatomy can be appreciated with a higher resolution, wide angle OCT scan (Figure 2).
 |
|
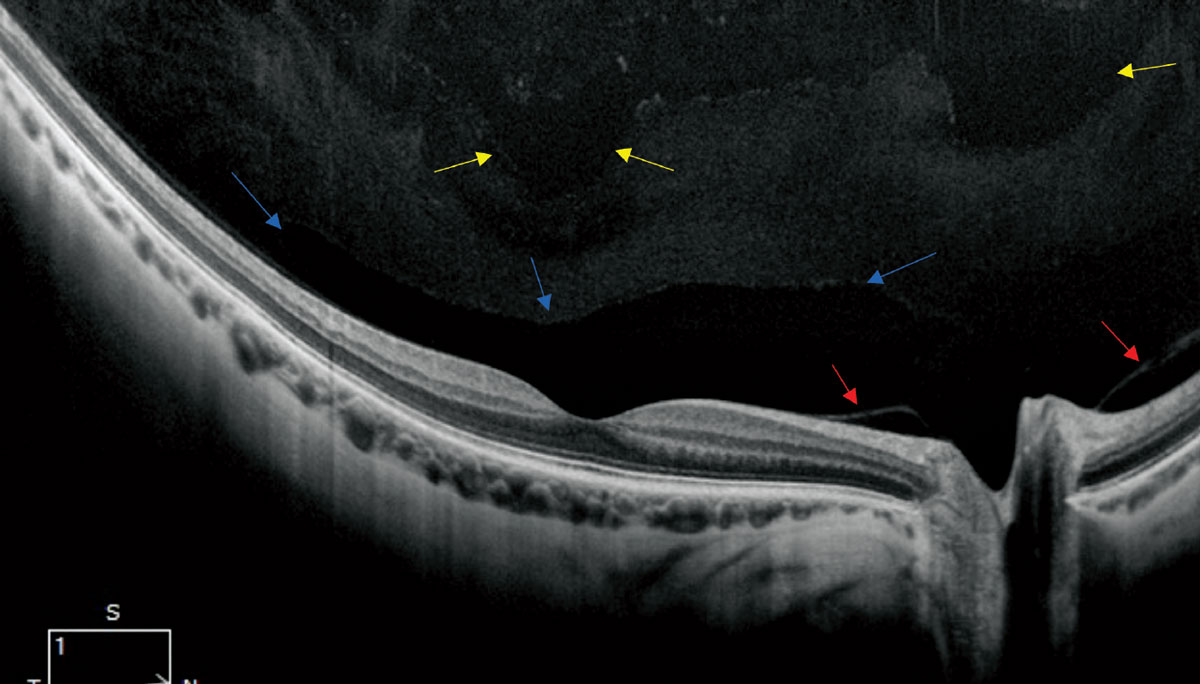
Fig. 2. A high-resolution single line scan OCT of a healthy, myopic 30-year-old (12mm HD 1-Line Raster 100x, Zeiss Cirrus OCT). The blue arrows point to the normal anatomic bursa premacularis. The red arrows reveal a non-pathologic release of the posterior hyaloid. In the more anterior aspect of the image, there is evidence of liquefaction noted by the optically empty voids noted by the yellow arrows. Click image to enlarge. |
Types of Vitreous Opacities
Whether physiologic (e.g., floaters) or pathologic, these impact the inherent structural translucency of the vitreous. An opacity may be clinically subtle or apparent and likewise for patients either visually symptomatic or asymptomatic.
Floaters. The most encountered of all vitreous opacities are primary vitreous floaters, also known as by the Greek-derived term myodesopsia. Most floaters occur as a result of increasing liquefaction with age, myopic vitreopathy or in PVD. With age, collagen fibrils aggregate into fibers that can scatter light, resulting in symptomatic floaters.10 Descriptions vary from patient to patient and include cobwebs, lines or hairs to dots, spots, gnats and flies. While floaters are a minor nuisance for most, some are chronically visually significant in terms of the visual disturbance and/or impact on contrast sensitivity.11
Treatment of chronically symptomatic floaters has increased due in part to development of smaller-gauge instruments in vitrectomy as well as recognition of the visual burden caused to some individuals. With proper patient selection, a standard three-port pars plana vitrectomy has proven successful in high rates of patient satisfaction, as well as low incidence of complication.12-14 To reduce complication risk further, retina surgeons can employ a limited or core vitrectomy, removing just the central vitreous gel.
Sebag and colleagues performed a limited vitrectomy using 25-gauge instruments for symptomatic floaters. They found quantitative echodensity decreased by 94% as well as improvement in visual function questionnaire, visual acuity and contrast sensitivity function.12
An alternative procedure is YAG vitreolysis, which acts as a photodisruptor, breaking down a floater into much smaller opacities.15 The targeted floater must be clinically appreciable, like a Weiss ring, and anterior enough from the retina to reduce risk of collateral damage.16 More than one treatment session may be needed to reduce symptoms, and even then there are some patients that remain symptomatic from the smaller opacities.8
PVD. This process occurs as gel liquefaction increases and the adhesion at the vitreoretinal interface weakens. Understanding of the vitreous changes leading up to PVD have changed dramatically, largely due to advances in imaging. PVD is now recognized as an insidious process occurring over years to decades until the development of complete PVD with vitreous release at the optic nerve.17,18 Long prior to vitreoretinal separation, there is evidence of lacunae development and vitreo-schisis, a lamellar separation in the cortex.19 Vitreoschisis has been noted as early as the third decade at the mid-peripheral interface.20
 |
|
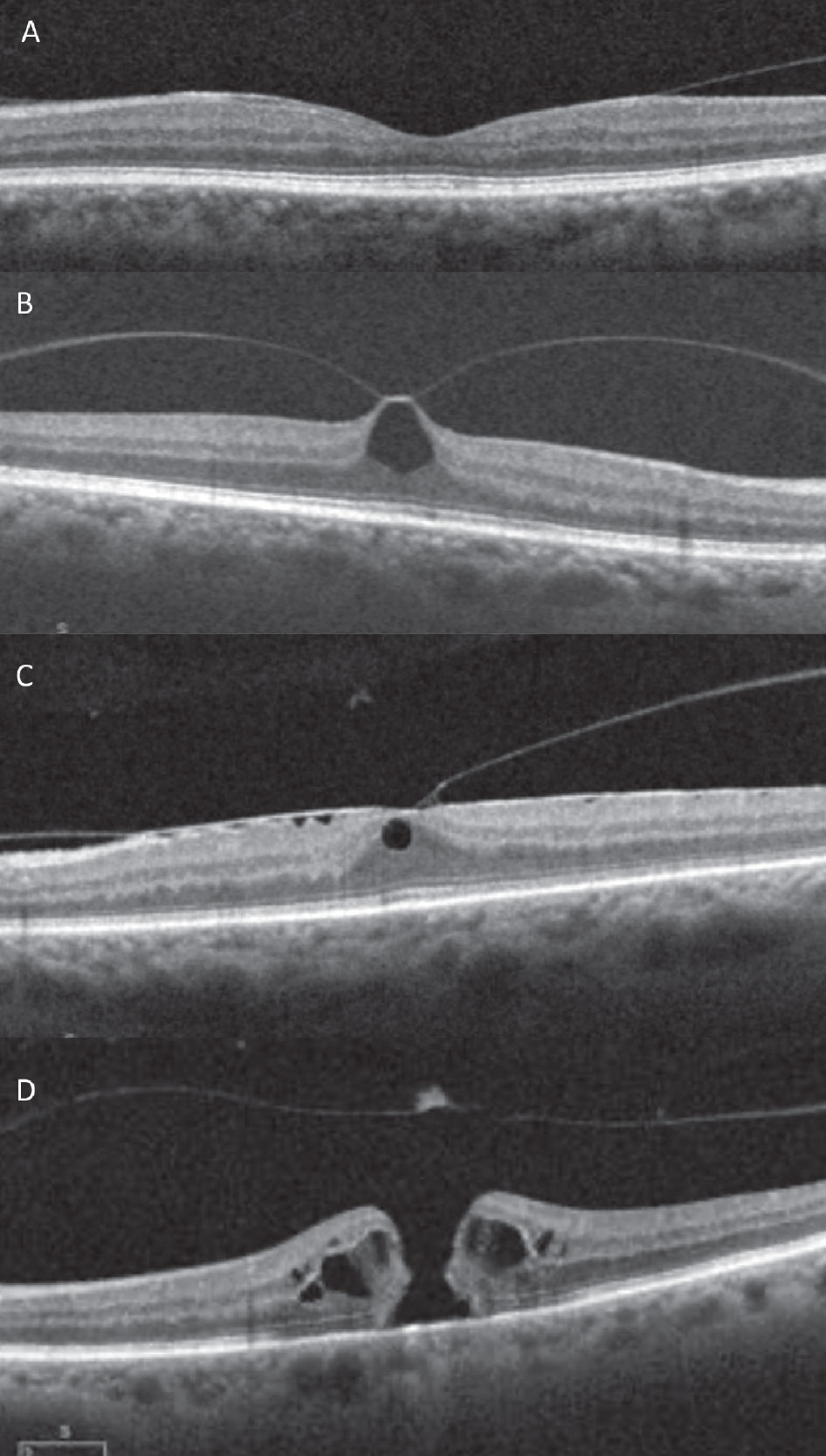
Fig. 3. SD-OCT images of the vitreomacular interface. (A) VMA, (B) focal VMT with pseudocyst, (C) broad VMT with ERM, (D) medium full thickness macular hole with operculum and posterior hyaloid release. Click image to enlarge. |
Recent studies using montaged wide-angle OCT imaging demonstrated evidence of separation in the mid-peripheral retina first, contrary to previous thought that the vitreous releases first in the perimacula.20,21 This is followed by release in the macula, maintaining adhesion at the fovea. On OCT, this finding is known as vitreomacular adhesion, evidenced in image Figure 3a.22 Subsequently, the fovea will release, followed eventually by the optic nerve, considered a complete PVD, which may be evidenced clinically with a Weiss ring.
If gel liquefaction occurs faster than vitreoretinal dehiscence, termed anomalous PVD, multiple complications can occur. Across the retina, concomitant diseases such as proliferative retinopathy, diabetic macular edema and exudative AMD can be aggravated. In the periphery, the traction from anomalous PVD can result in retinal tear or detachment. The peak risk for rhegamatogenous retinal detachment is in the 50 to 70 age group as a complication of PVD.23 For an acutely symptomatic PVD, there is a risk for a concomitant retinal break, with or without detachment, signifying need for a thorough retinal exam.
A recent study by Seider and colleagues found an incidence of retinal breaks or retinal detachment in 9.4% in a primary eye care setting.24 Prior reports suggested higher but have been out of retinal specialty settings. Highest risk characteristics for a simultaneous or delayed retinal break include concomitant vitreous hemorrhage, greater than 3D of myopia, lattice degeneration and a history of break in the other eye.24-26 Symptoms of photopsia during PVD are often a part of the process and not viewed as a risk factor.24 Though uncommon, delayed retinal break has been reported in 1.8% to 2.6% of acute PVDs.24-26 As such, follow-up is always recommended after an acute PVD, most suggesting four to six weeks. For those with risk factors, an additional follow-up may be considered.25
At the vitreomacular interface, the antero-posterior force of traction from anomalous PVD can lead to vitreomacular traction (VMT). Unlike VMA, VMT demonstrates a tractional anatomic change to the contour of the fovea without a full-thickness retinal defect.22 With focal VMT, this may appear as a loss of foveal contour or pseudocyst formation.22 Broad VMT, a wider area of attachment, more likely will show retinal thickening, cystoid macular edema or fovealschisis.22
While some VMT will spontaneously resolve, other patients may develop symptoms such as metamorphopsia or decreased vision, and some will progress on to macular hole. An estimated 5% to 12% of VMT will progress on to full thickness macular hole.27-29
Those asymptomatic or with minimal symptoms can be observed. However, in the development of visually significant symptoms or with progression to macular hole, the most successful treatment is vitrectomy. A small (<250µm) macular hole has a near 100% closure rate with vitrectomy and gas tamponade.30,31 With vitrectomy, gas tamponade and internal limiting membrane peel are often needed to secure a return to proper foveal contour. An alternative treatment option was the intravitreal injection of Jetra (ocriplasmin, ThromboGenics), which produced proteolytic activity to the proteins at the interface to induce PVD. The drug’s high cost, adverse effects and at best 50% success rate likely contributed to its discontinuation in 2020.32 The concept of pneumatic vitreolysis, injection of a small gas (C3F8 or SF6) bubble, has gained interest showing highly successful release of VMT in small non-controlled trials.33,34 This led to DRCR Retina Network clinical trials AG (VMT) and AH (macular hole), which were ultimately discontinued due to high rates of retinal tear and detachment.35 To date, vitrectomy remains the only treatment option for VMT and macular hole.
Pigment. Sometimes during anomalous PVD, pigmented cells, known as Shafer’s sign or “tobacco dust” sign, can be observed in the anterior vitreous. These cells are usually brown in color and are thought to represent released retinal pigment epithelial cells into the vitreous from a retinal break.8 Multiple studies have shown that the presence of a Shafer’s sign is almost pathognomonic for a retinal break or rhegmatogenous detachment and a clinician should be on high alert if noted on an examination.
One United Kingdom study found that approximately 95% of patients with an acute PVD and a positive Shafer’s sign had a retinal break.36 Additionally, out of 113 patients referred for a retinal detachment repair, 111 had vitreous pigment.36 Because these cells are located within the anterior vitreous, they are best viewed at the slit lamp by using a narrow, bright beam and focusing just posterior to the lens. It may be helpful to have the patient look up or down in order to better observe the cells moving within the vitreous.
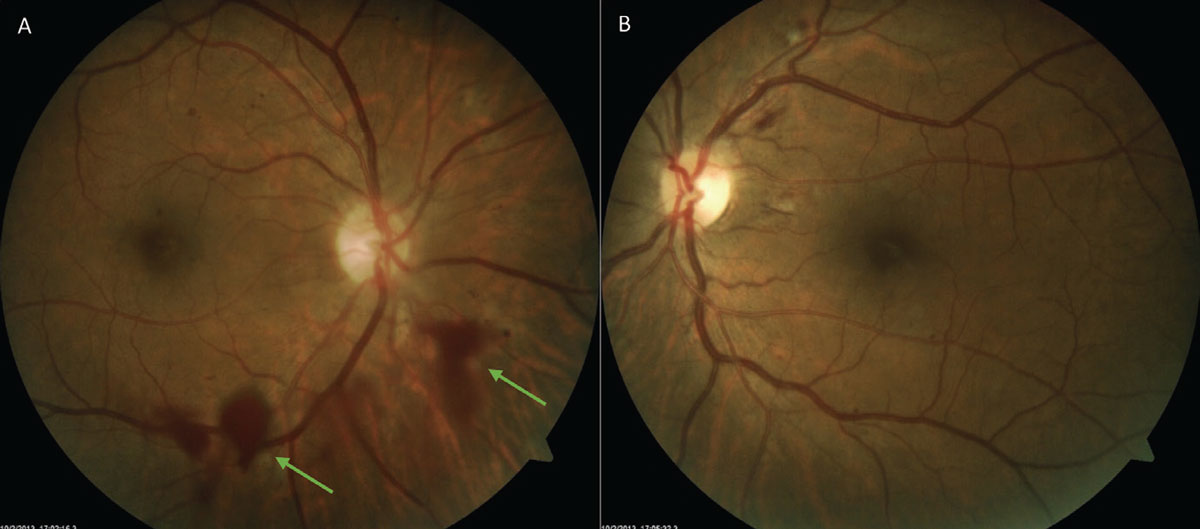
Hemorrhage. Acute-onset floaters may be the only presenting symptom with an acute vitreous hemorrhage, which makes it a challenge to discern from history alone. Some will notice a red hue to the new floaters. Others, particularly with a dense hemorrhage, may not notice floaters at all but rather experience a sudden loss of vision. Similarly, vitreous hemorrhage can vary clinically, appearing either diffuse or localized (Figure 4). A subtle vitreous hemorrhage may be most appreciable inferiorly with settling of red blood cells. Blood confined to the sub-hyaloid space will appear boat-shaped. In a dense vitreous hemorrhage, the media becomes hazy making visualization of the retina challenging. Thorough examination of the fellow eye is key to revealing clues toward the etiology. B-scan may also be necessitated in cases of poor retinal view. When blood is noted in the vitreous, it often boils down to one of two causes: abnormal vessels from proliferative retinopathy or a rupture of normal retinal blood vessels.37,38
 |
|
Fig. 4. An inferior localized vitreous hemorrhage (A) due to neovascularization elsewhere from diabetic retinopathy. Note the hazy quality from the hemorrhage when compared with OS (B) with moderate nonproliferative diabetic retinopathy. Click image to enlarge. |
Proliferative retinopathy. This is the most common cause of blood within the vitreous and refers to the growth of new blood vessels within the eye in response to capillary nonperfusion.38 This neovascularization is most often seen in diabetic retinopathy, but also occurs in numerous other conditions such as retinal vascular occlusions, sickle cell disease and retinopathy of prematurity.38 In an ischemic state, VEGF and other cytokines are released within the eye causing the formation of these abnormal blood vessels at the vitreoretinal interface. However, these new vessels are fragile and prone to shearing and leakage when they experience traction from the vitreous, which can ultimately lead to a vitreous hemorrhage.39
Both OCT and OCT-A imaging can be helpful in diagnosing neovascularization and demonstrating its relationship with the vitreous. On an OCT B-scan, neovascularization will breach the internal limiting membrane, extend into the vitreous and can take on a number of appearances (Figure 5).40 On OCT-A, extension of these vessels into the vitreous cavity can be seen on the vitreoretinal interface slab, one that should normally be black and devoid of blood flow. Flow within these vessels is also evidenced by using the flow overlay tool on the corresponding B-scan.
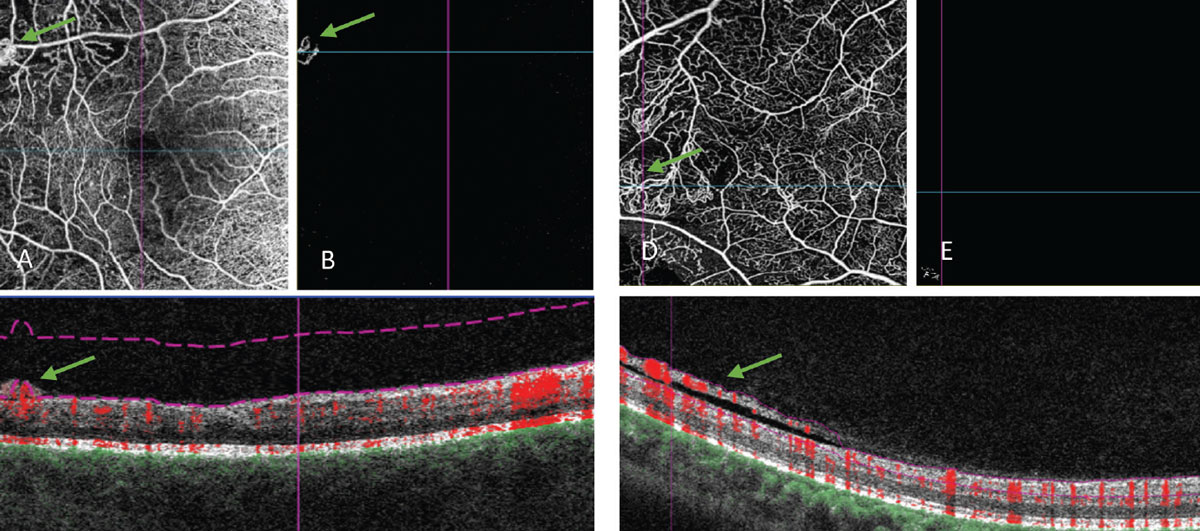 |
Fig. 5. OCT angiography of retinal neovascularization. (A) An angiogram of the retinal vessels. (B) VRI slab that segments out the vitreoretinal interface. This scan should normally be black, as this area is void of blood vessels in a healthy individual. (C) Corresponding B-scan, where you can see the abnormal vessels fully protruding past the posterior hyaloid and into the vitreous. The flow overlay tool shows red coloring within these vessels corresponding to active movement or flow within. (D, E and F) Another OCT-A of flat neovascularization that extends along the posterior vitreous surface. (F) Because of how it is improperly segmented in the B-scan (purple lines), the abnormal blood vessels do not show in the VRI slab. Click image to enlarge. |
Hemorrhagic PVD. After proliferative retinopathy, the next most common cause of vitreous hemorrhage is from a disruption to normal blood vessels either via PVD, retinal break or trauma.37,38
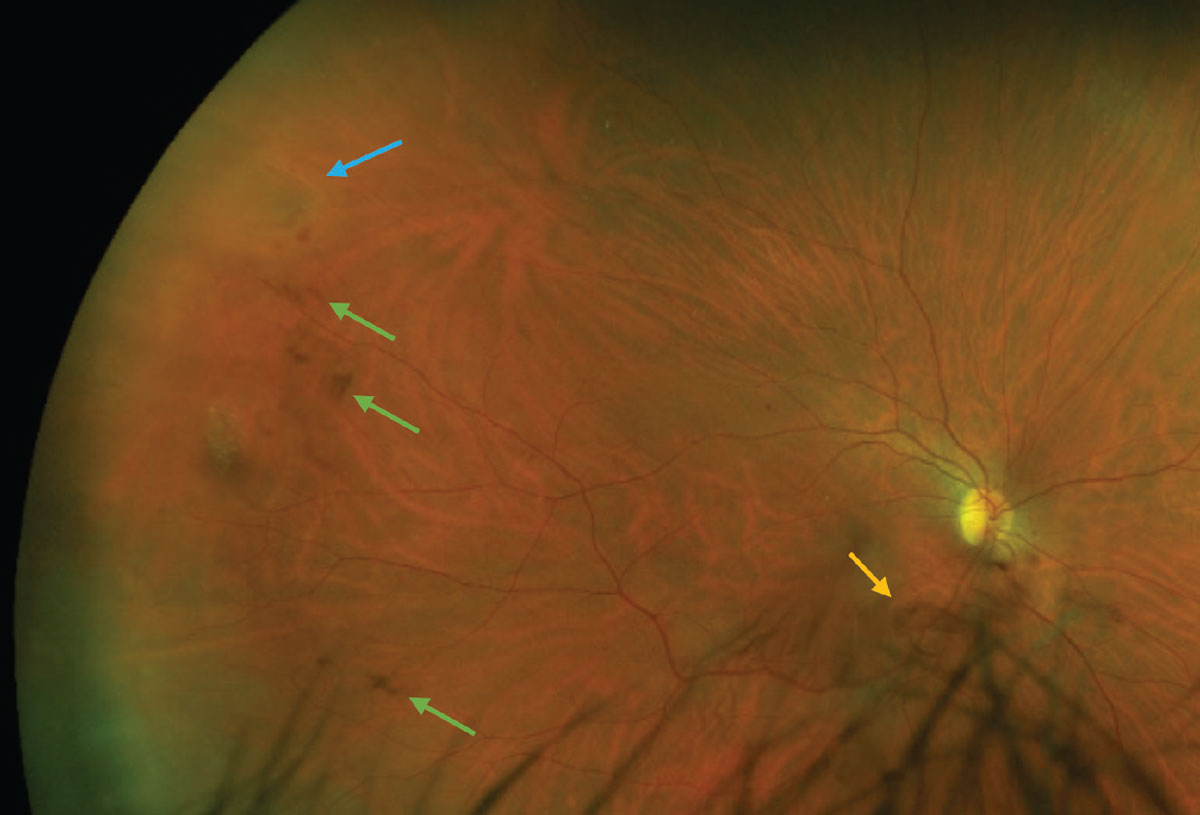
When vitreous hemorrhage develops as a result of the traction that occurs during PVD, it signals a high risk of concomitant or delayed retinal break approximately 60% to 70% risk.38,41 As such, either closer observation or a retina specialist referral is indicated depending on your comfort level and ability to visualize the retina. Also, be mindful that patients on anticoagulants like aspirin, coumadin and warfarin have a higher propensity for vitreous hemorrhage from PVD.42
 |
|
Fig. 6. A patient with an acutely symptomatic PVD and concomitant retinal horseshoe tear (blue arrow) and vitreous hemorrhage (green arrows). The Weiss ring can be seen inferior to the optic nerve head (yellow arrow). Click image to enlarge. |
In most cases, hemorrhage will clear on its own, typically at the rate of approximately 1% per day.37 Younger eyes with a more gelatinous vitreous take longer to clear.37 Vitrectomy indications are dependent on etiology. This procedure may be considered in the case of dense hemorrhage with poor retinal visualization or non-clearing hemorrhage (two to six months’ duration) to reduce risk of complications.38,41
Asteroid hyalosis. This is an age-related condition in which small, yellow-white refractile bodies are suspended throughout an intact vitreous. These opacities are made up of calcium-phospholipid complexes and the exact etiology remains unknown.43 It’s fairly common, found in about 1% to 2% of the population, and its incidence increases with age.44,45 It is usually unilateral but can be bilateral and in the past, it was thought to have an association with systemic conditions, including diabetes, hypertension and hyperlipidemia. It should be noted that recent epidemiologic studies have not confirmed a significant correlation.44-46
Unlike the other vitreous opacities described here, asteroid hyalosis has little effect on vision and affected patients are generally asymptomatic.43 Sometimes, it can be difficult for a practitioner to accurately view and assess the fundus, and other techniques such as fluorescein angiography, B-scan, OCT and OCT-A should be used. For the few cases that are symptomatic or hinder a necessary view of the posterior pole, a vitrectomy may be indicated.
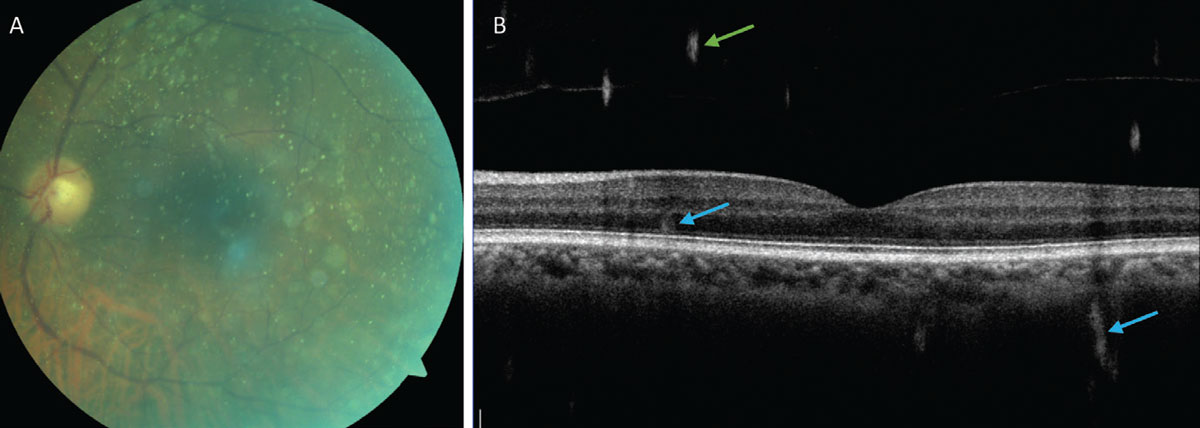 |
Fig. 7. (A) Fundus photo showing asteroid hyalosis and demonstrating the limited visibility of the retina. (B) An OCT B-scan of the retina with asteroid bodies visible in the vitreous (green arrows) and artifacts from the asteroid bodies within the retina and choroid (blue arrows). Click image to enlarge. |
Similar in appearance to asteroid hyalosis, synchysis scintillans also presents as yellow-white highly refractile bodies within the vitreous. However, this entity is made up of cholesterol deposits and is a degenerative process found in severely diseased eyes.47,48 Unlike asteroid hyalosis, synchysis scintillans opacities move freely within a liquified vitreous and will settle inferiorly when the eye is not moving.43
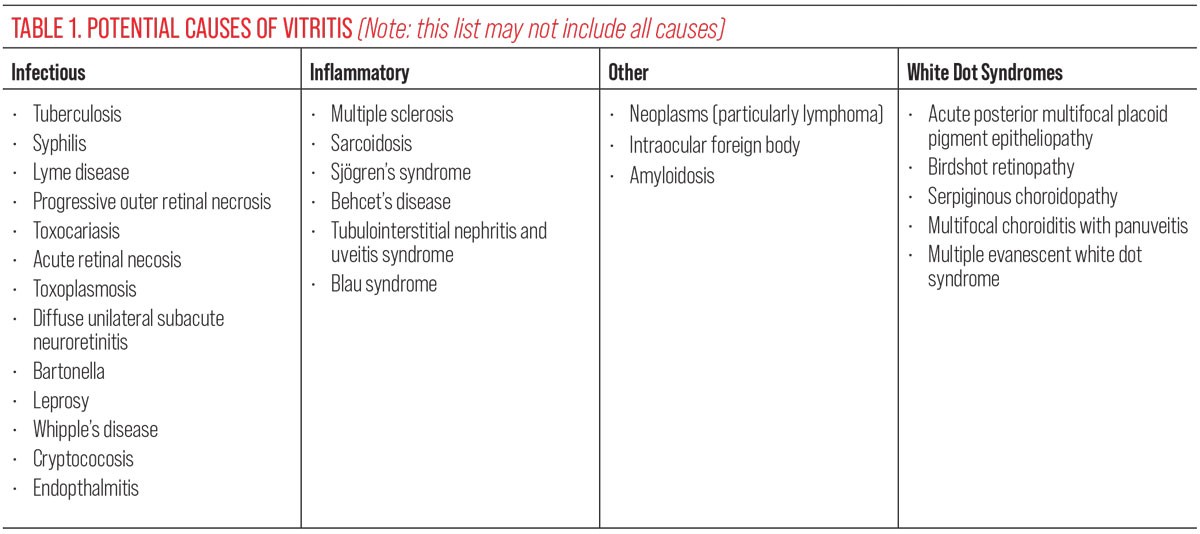
Vitritis/white blood cells. Acute-onset floaters may be the primary presenting symptom of ocular inflammation or infection, particularly in the case of vitritis, clinically evidenced by white blood cells within the vitreous. Whether physiologic, like floaters, or pathologic, an opacity impacts the inherent structural translucency. They may be clinically subtle or apparent and likewise for patients either visu-ally symptomatic or asymptomatic. Causes include infection, inflammation, uveitis masqueraders, white dot syndromes and idiopathic disease (Table 1).
 |
The appearance of these cells is also highly variable depending on the specific cause and length of time they have been present. When a patient is newly symptomatic for floaters, cells may be small, few in number and difficult to appreciate. Cells can also aggregate inferiorly and be considered “snowballs” or spread across the ora serrata and pars plana in what is known as “snowbanking.”50 Depending on the specific cause, deeper structures of the eye such as the retina or choroid are often involved as well.
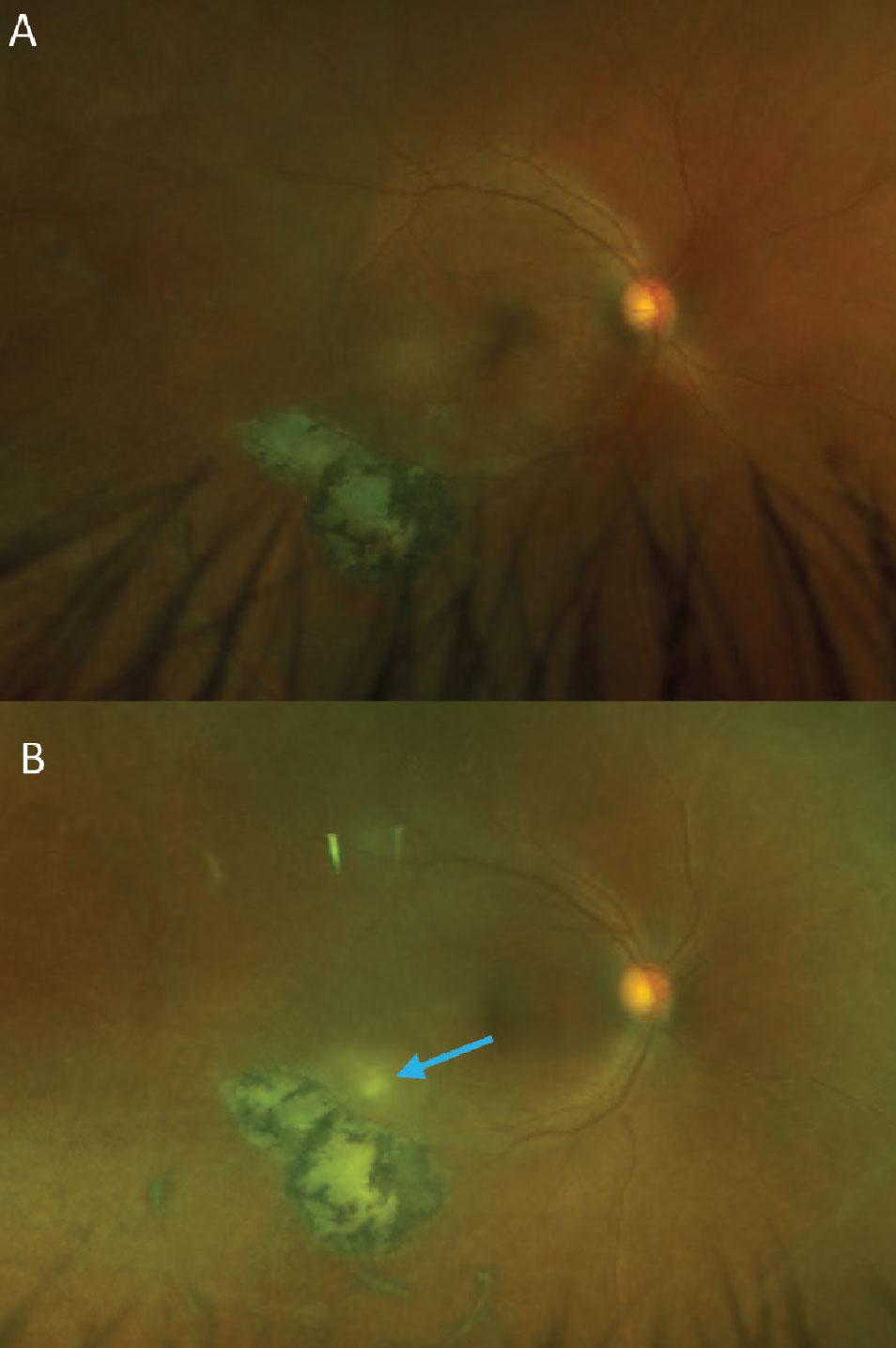
Because vitritis is a nonspecific finding, workup includes a very detailed history, taking into account patient demographics, a full history of present illness and a comprehensive medical history. Bloodwork is often necessary for a definitive diagnosis, and the amount of tests ordered can be considerable. Often, this list can be narrowed down based on the history and physical examination. Take for example, a patient who presented with acute floaters (Figure 8). The clinical exam revealed a classic appearance consistent with active ocular toxoplasmosis gondii chorioretinitis infection. Toxoplasmosis is the most common cause of posterior uveitis and is associated with the classic “headlights in fog” appearance, with a new focal, white lesion visible through an often dense vitritis.50 Bloodwork was positive for elevated toxoplasmosis antibody titers and negative for HIV, which is important to rule out, as reactivation of these lesions can occur in an immunocompromised state.
 |
|
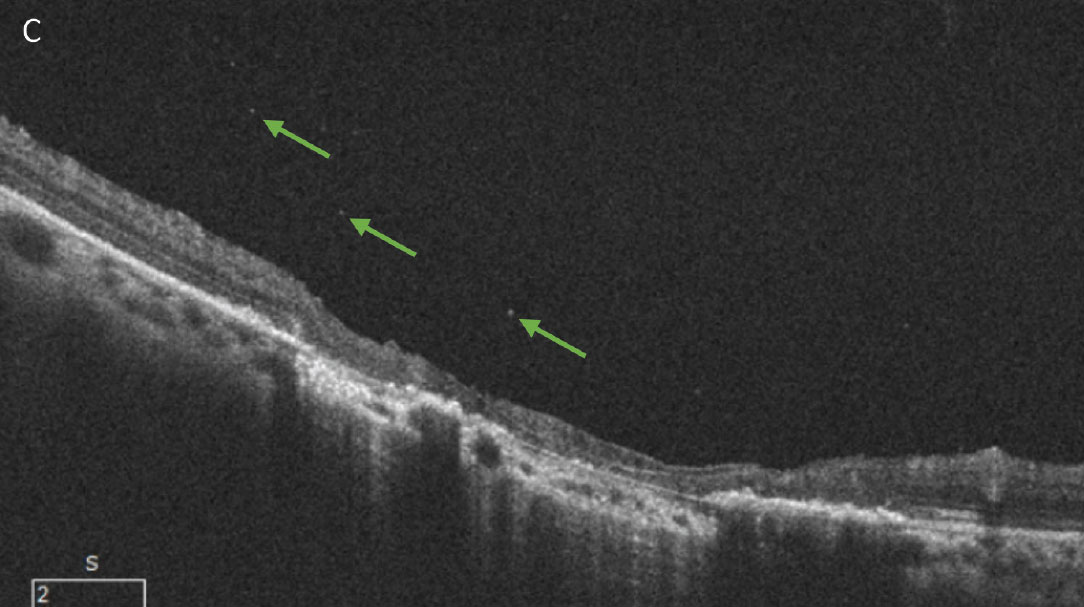
Fig. 8. (A) A patient with an inactive toxoplasmosis gondii chorioretinal scar. (B) The same patient with a reactivated infection. The new, white retinal lesion can be seen just superior to the existing scar. A mild vitritis is evident from the haziness of the photo compared to the original. Small cells can be seen on close inspection of the optos photo as well as within the vitreous on the OCT B-scan (green arrows, C). This patient was seen very soon after acutely noticing new floaters in their vision and the vitritis in this case is more subtle than is often seen with toxoplasmosis. Click image to enlarge. |
 |
| Click image to enlarge. |
Takeaways
While often overlooked, the vitreous via its unique aging process, as well as its interaction with the retina, is susceptible to a variety of different conditions. Although bothersome, many vitreous opacities are harmless and need only patient education and yearly monitoring. On the other hand, entities such as pigment, hemorrhage or white blood cells within the vitreous can be cause for serious concern. It is our role to be able to differentiate the benign from the harmful and successfully manage accordingly.
Dr. Bedwell is a clinical associate professor at Indiana University School of Optometry. She is a fellow of the American Academy of Optometry and the Optometric Retina Society, as well as a member of the American Optometric Association. She serves as editor of the Optometric Retina Society’s e-newsletter.
Dr. Krenk is an assistant clinical professor at Indiana University School of Optometry, where she teaches optometry students and see patients at the Indianapolis Eye Care Center. She is also a fellow of the American Academy of Optometry and a member of the American Optometric Association. They have no financial interests to disclose.
1. Foos RY, Wheeler NC. Vitreoretinal juncture: synchysis senilis and posterior vitreous detachment. Ophthalmology. 1982;89(12):1502-12. 2. Balazs EA, Flood MT. Age-related changes in the physical and chemical structure of human vitreous. Third International Congress of Eye Research. Osaka. 1978. 3. Fincham GS, James S, Spickett C, et al. Posterior vitreous detachment and the posterior hyaloid membrane. Ophthalmology. 2018;125(2):227-36. 4. Snead MP, Snead DR, Richards AJ, et al. Clinical, histological and ultrastructural studies of the posterior hyaloid membrane. Eye (Lond). 2002;16(4):447-53. 5. Snead MP, Snead DR, James S, Richards AJ. Clinicopathological changes at the vitreoretinal junction: posterior vitreous detachment. Eye (Lond). 2008;22(10):1257-62. 6. Balazs EA, Denlinger JL. Aging changes in the vitreous. In: Dismukes K and Sekular R (eds). Aging and human visual function. Alan R Liss, Inc.: New York. 1982:45-57. 7. Le Goff MM, Bishop PN. Adult vitreous structure and postnatal changes. Eye (Lond). 2008;22(10):1214-22. 8. Milston R, Madigan MC, Sebag J. Vitreous floaters: etiology, diagnostics, and management. Surv Ophthalmol. 2016;61(2):211-27. 9. Mamou J, Wa CA, Yee KMP, et al. Ultrasound-based quantification of vitreous floaters correlates with contrast sensitivity and quality of life. Invest Ophthalmol Vis Sci. 2015;56(3):1611-7. 10. Sebag J, Balazs EA. Morphology and ultrastructure of human vitreous fibers. Invest Ophthalmol Vis Sci. 1989;30(8):1867-71. 11. Sebag J. Vitreous and vision degrading myodesopsia. Prog Retin Eye Res. 2020;79:100847. 12. Sebag J, Yee KMP, Nguyen JH, Nguyen-Cuu J. Long-term safety and efficacy of limited vitrectomy for vision degrading vitreopathy resulting from vitreous floaters. Ophthalmol Retina. 2018;2(9):881-9. 13. Ivanova T, Jalil A, Antoniou Y, et al. Vitrectomy for primary symptomatic vitreous opacities: an evidence-based review. Eye (Lond). 2016;30(5):645-55. 14. Fink S, Kumar JB, Cunningham MA. Small-gauge pars plana vitrectomy for visually significant vitreous floaters. J Vitreoretin Dis. 2021;5(3):247-50. 15. Sebag J. Methodological and efficacy issues in a randomized clinical trial investigating vitreous floater treatment. JAMA Ophthalmol. 2018;136(4):448. 16. Katsanos A, Tsaldari N, Gorgoli K, et al. Safety and efficacy of YAG laser vitreolysis for the treatment of vitreous floaters: an overview. Adv Ther. 2020;37(4):1319-27. 17. Johnson MW. Posterior vitreous detachment: evolution and complications of its early stages. Am J Ophthalmol. 2010;149(3):371-82. 18. Johnson MW. Perifoveal vitreous detachment and its macular complications. Trans Am Ophthalmol Soc. 2005;103:537-67. 19. Sebag, J. Vitreoschisis. Graefes Arch Clin Exp Ophthalmol. 208;246(3):329-32. 20. Tsukahara M, Mori K, Gehlbach PL, Mori K. Posterior vitreous detachment as observed by wide-angle OCT imaging. Ophthalmology. 2018;125(9):1372-83. 21. Kraker JA, Kim JE, Koller EC, et al. Standard 6mm compared with widefield 16.5mm OCT for staging of posterior vitreous detachment. Ophthalmol Retina. 2020;4(11):1093-102. 22. Duker JS, Kaiser PK, Binder S, et al. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction and macular hole. Ophthalmology. 2013;120(12):2611-9. 23. El-Abiary M, Shams F, Goudie C, Yorston D. The Scottish RD survey 10 years on: the increasing incidence of retinal detachments. Eye. 2023;37(7):1320-4. 24. Seider MI, Conell C, Melles RB. Complications of acute posterior vitreous detachment. Ophthalmology. 2022;129(1):67-72. 25. Uhr JH, Obeid A, Wibbelsman TD, et al. Delayed retinal breaks and detachments after acute posterior vitreous detachment. Ophthalmology. 2020;127(4):516-22. 26. Vangipuram G, Li C, Li S, et al. Timing of delayed retinal pathology in patients p[resenting with acute posterior vitreous detachment in the IRIS Registry (Intelligent Research in Sight). Ophthalmol Retina. 2023;S2468-6530(23):00154-9. 27. Errera MH, Liyanage SE, Petrou P, et al. A study of the natural history of vitreomacular traction syndrome by OCT. Ophthalmology. 2018;125(5):701-7. 28. Stalmans P. A retrospective cohort study in patients with tractional diseases of the vitreomacular interface (ReCoVit). Graefes Arch Clin Exp Ophthalmol. 2016;254(4):617-28. 29. Petrou P, Chalkiadaki E, Errera MH, et al. Factors associated with the clinical course of vitreomacular traction. J Ophthalmol. 2020;2020:9457670. 30. Ip MS, Baker BJ, Duker JS, et al. Anatomical outcomes of surgery for idiopathic macular hole as determined by optical coherence tomography. Arch Ophthalmol. 2002;120(1):29-35. 31. Ullrich S, Haritoglou C, Gass C, et al. Macular hole size as a prognostic factor in macular hole surgery. Br J Ophthalmol. 2002;86:390-3. 32. Morescalchi F, Gambicorti E, Duse S, et al. From the analysis of pharmacologic vitreolysis to the comprehension of ocriplasmin safety. Expert Opin Drug Saf. 2016;15(9):1267-78. 33. Chan CK, Crosson JN, Mein CE, Daher N. Pneumatic vitreolysis for relief of vitreomacular traction. Retina. 201737(10):1820-31. 34. Özdemir HB, Özdek Ş, Hasanreisoğlu M. Pneumatic vitreolysis for the treatment of vitreomacular traction syndrome. Turk J Ophthalmol. 2019;49(4):201-8. 35. Chan CK, Mein CE, Glassman AR, et al; DRCR Retina Network. Pneumatic vitreolysis with perfluoropropane for vitreomacular traction with and without macular hole: DRCR Retina Network Protocols AG and AH. Ophthalmology. 2021;128(11):1592-603. 36. Tanner V, Harle D, Tan J, et al. Acute posterior vitreous detachment: the predictive value of vitreous pigment and symptomatology. Br J Ophthalmol. 2000;84(11):1264-8. 37. Spraul CW, Grossniklaus HE. Vitreous hemorrhage. Surv Ophthalmol. 1997;42(1):3‑39. 38. Shaikh N, Srishti R, Khanum A, et al. Vitreous hemorrhage - causes, diagnosis, and management. Indian J Ophthalmol. 2023;71(1):28-38. 39. Arya M, Sorour O, Chaudhri J, et al. Distinguishing intraretinal microvascular abnormalities from retinal neovascularization using OCT-A. Retina. 2020;40(9):1686-95. 40. Pan J, Chen F, Chen D, et al. Novel three types of neovascularization elsewhere determine the differential clinical features of proliferative diabetic retinopathy. Retina. 2021;41(6):1265-74. 41. Pighin MS, Berrozpe C, Jürgens I. Outcome of acute nontraumatic vitreous hemorrhage in healthy patients. Retina. 2020;40(1):87-91. 42. Witmer MT, Cohen SM. Oral anticoagulation and the risk of vitreous hemorrhage and retinal tears in eyes with acute posterior vitreous detachment. Retina. 2013;33(3):621-6. 43. Margo CE. Age-related diseases of the vitreous. In: Cavallotti CAP, Cerulli L, eds. Age-related changes of the human eye. Aging Medicine. Humana Press; 2008:166-8. 44. Mitchell P, Wang MY, Wang JJ. Asteroid hyalosis in an older population: the Blue Mountains Eye Study. Ophthalmic Epidemiol. 2003;10(5):331-5. 45. Fawzi AA, Vo B, Kriwanek R, et al. Asteroid hyalosis in an autopsy population: The University of California at Los Angeles (UCLA) experience. Arch Ophthalmol. 2005;123(4):486-90. 46. Moss SE, Klein R, Klein BE. Asteroid hyalosis in a population: the Beaver Dam eye study. Am J Ophthalmol. 2001;132(1):70-5. 47. Wand M, Smith TR, Cogan DG. Cholesterosis bulbi: the ocular abnormality known as synchysis scintillans. Am J Ophthalmol. 1975;80(2):177-83. 48. Park J, et al. A case of cholesterosis bulbi with secondary glaucoma treated by vitrectomy and intravitreal bevacizumab. Korean J Ophthalmol. 2011;25(5):362-5. 49. Kanski, JJ. Clinical Ophthalmology: a systematic approach (6th ed.). Butterworth Heinemann, Elsevier Science; 2007. 50. Bagheri N, Wajda B, Calvo CM, Durrani AK. (Eds.). The Wills Eye Manual (7th ed.). Lippincott Williams and Wilkins; 2017:346-54. |