Imagine this: An 8-year-old boy presents to your office complaining of pain in his left eye. His conjunctiva is obviously inflamed. In addition, his cheeks are flushed, and he has a raspy cough. The Difference Between an Epidemic and a Pandemic An epidemic is a disease that affects a disproportionately large number of individuals within a population, community or region at the same time. So, an epidemic may be isolated to one community. A pandemic is an epidemic that spreads worldwide or at least over a great region. Three conditions are necessary to create the potential for an influenza pandemic:
You take his history, and he tells you his eye began hurting the evening before last. You ask him about the day that lead up to that evening, and he excitedly tells you his third-grade class visited a farm three days prior. He denies any injury to the eye. Upon further questioning, the boy reports that his throat started feeling sore upon waking this morning. Also, he tells you that his arms and legs started feeling achy before he went to sleep last night. As youre about to examine the boy, one of your staff members interrupts to tell you in private that she has received seven calls thus far from parents reporting similar ocular pain in their children and in themselves.
While this scenario may seem like an outbreak of run-of-the-mill conjunctivitis, it is actually the beginning of an avian flu pandemic, something you may be faced with in the future (see The Difference Between an Epidemic and a Pandemic, below).
1. Rare emergence of a new influenza subtype not previously circulated in humans.
2. The new subtype must be capable of inducing disease in the human host.
3. The subtype must be readily transmitted from human to human.
There are six pandemic phases. The inter-pandemic period includes phases 1 and 2.
Phase 1 is at the lowest risk of a pandemic. No new influenza subtypes are detected in humans, but subtypes capable of causing disease in humans may be present in animals.
Phase 2 means that no new influenza subtypes are detected in humans, but subtypes are circulating in animals. These may pose a risk to humans.
The pandemic alert period includes Phases 3, 4 and 5. Phase 3 conditions, present at the time of this article, are met when human infections with a new subtype have oc-curred, but no human-to-human spread or rare close-contact human-to-human transmissions have occurred. Phase 4 consists of small, highly localized clusters of limited human-to-human transmission, which suggests that the virus is not well adapted to humans. Phase 5 has larger clusters, but human-to-human transmission is still localized, suggesting that the virus has not fully adapted for optimum human-to-human transmission. Still, it is considered a substantial pandemic risk. Phase 6 is the pandemic period, with sustained transmission to the general population.1
The World Health Organization (WHO) projects that an avian influenza pandemic is expected to exhaust health-care resources, interrupt commerce and have a major economic impact.2 As a result, the United States, along with many other countries and organizations, is actively preparing for the possibility of such a pandemic. Indeed, Congress has appropriated $334 million to support the global campaign against bird flu and $2.3 billion to prepare domestically for a possible influenza pandemic.3,4
The last three global influenza pandemics occurred in 1918, 1957 and 1968. The 1918 Spanish flu pandemic killed about 500,000 people in the United States and 50 million worldwide.5 In 1957, the Asian flu killed about 70,000 in the United States, and the 1968 Hong Kong flu killed 34,000 people in the United States.5
Although rare, the avian flu has the potential to manifest itself as conjunctivitis. This means that you, the primary-care optometrist, may be the first health-care professional to see a patient infected with this dangerous illness. You may even be the first to encounter an infected patient who does not have conjunctivitis if the patient presents to you for his or her annual exam exhibiting flu-like symptoms.
Influenza Virus Types
There are three types of influenza viruses: A, B and C. Type C viruses are generally not a concern for humans because these infections are mild and do not cause epidemics or pandemics. Although very common, the type B influenza virus, normally found only in humans, is not a pandemic maker. Every year, 5% to 15% of the worlds population experiences an infection of seasonal influenzaa B influenza virus, which has a relatively low mortality rate. This is the influenza for which flu shots are made.
Influenza A, known as avian influenza, primarily infects birds, but may infect people, pigs, horses and other animals.6 When influenza A jumps the species barrier, it has the potential to create a new virus for which humans have no immunity, thus producing a pandemic. Influenza type A viruses, unlike influenza type B and C, are divided into subtypes based on two surface glycoproteins: hemagglutinin (HA) and neuraminidase (NA).
 |
| HPAI may kill 90% to 100% of infected chickens, while LPAI causes mild or no illnesses in infected chickens. Because the disease mortality in birds may not correlate to its effects in man, it is currently unclear how the distinction between HPAI and LPAI relates to the risk of serious illness in humans. |
There are 16 different HA subtypes and nine different NA subtypes.7 Influenza A viruses are classified according to their HA and NA proteins, such as H5N1, which has an HA 5 protein and an NA 1 protein. H5 and H7 subtypes of influenza type A are further classified as either highly pathogenic avian influenza (HPAI) or low pathogenic avian influenza (LPAI) based on the severity of the disease produced in birds. HPAI may kill 90% to 100% of infected chickens, while LPAI causes mild or no illnesses in infected chickens.
Because the disease mortality in birds may not correlate to its effects in people, it is currently unclear how the distinction between HPAI and LPAI relates to the risk of serious illness in humans.8 To complicate matters, LPAI may evolve into HPAI viruses. Because all past influenza pandemics in man have been caused by influenza A viruses, they are monitored closely by health officials.
Virus Strains and Subtypes
Influenza B viruses and subtypes of influenza A viruses are further classified into strains. New strains of influenza B and subtypes of influenza A viruses are constantly appearing and replacing older strains through two processes:
Antigenic drift. This is a slow process brought about from errors during viral replication in which one ribonucleic acid; adenine, guanine, cytosine, and uracil; is replaced by a different one. These errors cause point mutations, which change a single amino acid in the segments of the genome coding for two specific surface antigens (the NA and the HA segments).
Antigenic shift. The A virus, but not the B virus, also changes through a more dramatic and rapid genetic sorting process. Sorting occurs when the virus exchanges gene-tic materials in a host concurrently infected with another influenza virus. Sorting results in viruses that appear with new combinations of genes. This sudden and major change is known as antigenic shift and results in new influenza A subtypes. Antigenic shift has the potential to produce a new, highly virulent, easily transmitted virus. As new strains emerge, antibodies to the old strain are no longer effective against the new virus, and infections by the new strain may occur.9
The domestic swine has been referred to as a mixer, in which human and avian influenza viruses may exchange genetic material, thus returning to the bird, the human, or both. The H5N1 strain appears to infect the human without sorting taking place in the swine intermediary.10 This shift could also occur if a person suffering from a highly contagious influenza already in circulation in the human population contracts a new influenza A strain. Presently, poultry workers and health-care workers are vaccinated for the seasonal influenza to prevent reassortment of H5N1 with the B type, thus reducing the potential of forming a more dangerous subtype.11
Further virus changes called adaptive mutations occur while in the human host. These enhance the ability of the virus to pass more easily from human to human. This mechanism is thought to be responsible for the 1918 Spanish flu pandemic.12 If this new subtype (the mutated form) is introduced into the human population, which has no immunity to this new virus, the potential for a global pandemic is created.13
Avian Influenza Overview
The present suspect for an influenza virus pandemic is the H5N1. H5N1 has been occasionally transmitted human-to-human in very close contact, with one generation of contact, during the acute stage of illness. Therefore, at this time, it is not easily passed from human to human. Poultry-to-human infection apparently results from close contact with infected birds, feces of infected birds, nasal secretions of infected birds, infected blood or surfaces with infected bird material (typical environment of the poultry worker but not exclusively so) but in proportionally small numbers.14 Cooked poultry products are considered safe to eat.14
In the wild, fowl may not exhibit signs of the disease, but they readily transmit the virus to domestic birds. Wild waterfowl are the major natural reservoir for the virus. The backyard poultry industry in developing countries has been described as a major venue of wild-to-domestic bird spread and has the potential to move the virus across the species barrier to human poultry workers and beyond.15 The greater the number of geographical areas that have infected birds, the greater the opportunities for viral mutation or reassortment into a pandemic-capable virus.
 |
| The domestic swine has been referred to as a mixer, in which human and avian influenza viruses may exchange genetic material, thus returning to the bird, the human or both. |
So far, this strain has shown a limited ability for person-to-person transmission; however, this could change at any time. Because of the potential for large numbers of deaths, much attention has been given to the new strains of influenza A capable of infecting humans. The most worrisome at this time is the H5N1z genotype. As of August 7, 2006, the World Health Organization has officially recognized 233 human cases of H5N1 influenza, 135 of them fatal.16
The signs and symptoms of avian influenza H5N1 may be more variable than originally thought. During the 1997 outbreak in Hong Kong, the common manifestations were fever, headache, malaise, myalgia, sore throat, cough and rhinitis.5,13 Although uncommon, conjunctivitis was also reported.5,17 The 2004 epidemic in Vietnam was characterized by a severe influenza syndrome (signs and symptoms usually associated with the flu) with fever, cough, dypsnea and diarrhea.18 The 2004 epidemic in Thailand included fever, cough, dypsnea, sore throat, rhinorrhea and myalgia.
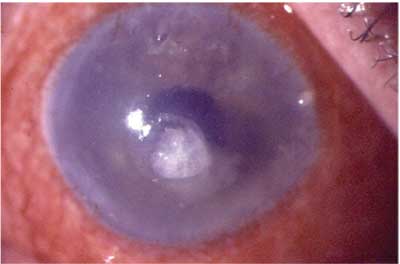 |
| Acute hemorrhagic conjunctivitis as seen in one avian influenza patient. |
Other strains of avian influenza, such as H7N3 and H7N7, have also manifested as conjunctivitis.19,20,21 Between February and May 2003, there was an outbreak of HPAI A virus of subtype H7N7 in the Netherlands. Of the 89 cases involved, 78 presented solely with conjunctivitis, and five presented with conjunctivitis and influenza-like illness.20 There was also one fatality associated with this outbreak.20 In addition, the University of Alabama at Birminghams Bioterrorism and Emerging Infections Education Web site lists conjunctivitis as a presenting symptom of avian influenza that should be monitored.8 Further, the April 8, 2004, Ontario Ministry of Health and Long-Term Care Important Health Notice asks that all patients who have conjunctivitis and a recent (within one week) exposure to infected or potentially infected poultry be reported and tested for avian influenza.22 (See The Three Pillars of U.S. National Strategy for Pandemic Influenza, below.)
|
The Three Pillars of the U.S. National Strategy For Pandemic Influenza |
| No pandemic in history has ever been halted by human intervention. Previous pandemics have spread across the globe within six to nine months. Now, a pandemic may encircle the globe within three months due to the smallness of the world from intercontinental travel. Containing this virus would be difficult because flu shots must be produced specifically for each strain of virus, and production and distribution of vaccines take much more than six months with the current chicken egg-based vaccines.23 Still, our government has a national strategy for pandemic influenza built upon three pillars:
Preparedness and communication. This pillar includes planning for a pandemic; communicating expectations and responsibilities; producing and stockpiling vaccines, antivirals and medical material; establishing distribution plans for vaccines and antivirals; advancing scientific knowledge, etc. |
Your Role in Infection Control
When a patient presents with red eye and flu-like symptoms, there are eight things you should do to protect yourself, your staff and your patients from the possibility of contracting avian influenza:
www.cdc.gov/ncidod/dhqp/pdf/Infdis/RespiratoryPoster.pdf, www.cdc.gov/flu/protect/pdf/covercoughhcp85x11.pdf, www.cdc.gov/ncidod/dhqp/pdf/ppe/ppeposter148.pdf.
Also, the CDC has formulated Interim Recommendations for Infection Control in Health-Care Facilities Caring for Patients with Known or Suspected Avian Influenza, which says, it is considered prudent to take all possible precautions to the extent feasible when caring for patients who have known or possible avian influenza.11 (See Summary of Infection Control Recommendations for Care of Patients with Pandemic Influenza, below.)
Cease wearing neckties or clothing that has bows or straps. A total of 47% of neckties worn by doctors are contaminated by bacteria.25,26 Consider the health of your patients and staff over fashion.
Consider isolating red eye patients in a separate reception area or room to avoid exposing other patients. Have your staff ask any patient coughing or exhibiting other signs of influenza to wear a mask and sit in a separate room or in an area separated by at least three feet from other patients in a common waiting area. (Three feet is the limit that small particles produced, when a person coughs or sneezes, float in the air.) (See Steps to Contain Respiratory Secretions, below.)
|
Steps to Contain Respiratory Secretions |
|
If you, your staff or your patient is coughing or exhibiting other signs of avian influenza: contain respiratory secretions by: Health-care facilities should ensure the availability of materials for adhering to respiratory hygiene/cough etiquette in waiting areas for patients and visitors: |
Use eye protection within three feet of the patient, and use at minimum a fit-tested National Institute of Occupational Safety and Health (NIOSH)-approved N-95 filtering face piece disposable respirator when entering the room. These respirators should be readily available at any medical supply store or Web site. Respirators should be used in the context of a complete respiratory protection program, as required by the Occupational Safety and Health Administration (OSHA). This includes training, fit-testing and fit-checking to ensure appropriate respirator selection and use. To be effective, respirators must provide a proper sealing surface on the wearers face. (Detailed information on a respiratory protection program is provided at www.osha.gov/SLTC/etools/respiratory/.)
Use gloves for all patient contact, and use disposable instruments if possible. For instance, your pen may become contaminated if you examine the patients eyes and then record your findings. For this reason, use a disposable pen, or have someone record your findings in order to prevent contamination of yourself and your records. (However, be sure this person is protected from contracting an infection from the patient.) Also, if you use electronic records, dont forget that the computer keyboard may become contaminated if you type before washing your hands.
Ask all patients who present with conjunctivitis if theyve recently traveled to a country that has cases of avian influenza in either humans or birds or if theyve had contact with people who have traveled to such countries within 10 days of the onset of symptoms.8,22 Because red eye from avian influenza is indistinguishable from other viral conjunctivitides, the case history is vital in differentiating patients. (Maps of areas with avian influenza in wild birds, domestic birds and humans are available at http://www.pandemicflu.gov)
Refer patients who have a red eye and in whom you suspect possible exposure to the avian influenza virus to a physician to test for avian influenza.
Once the red eye patient departs, use an EPA-registered hospital detergent-disinfectant on your sign-in pens, doorknobs, chair arms and any other object the patient touched. Also, discard any magazines these patients touch. Persons cleaning up after the patient should wear gloves. Remember, any object the patient touches may be contaminated with respiratory secretions if the patient coughed on his or her hands and did not immediately wash them.
|
Summary of Infection Control Recommendations for Care of Patients with Pandemic Influenza | |
| Component | Recommendations |
| Standard Precautions | See http://www.cdc.gov/ncidod/dhqp/gl_isolation_standard.html |
| Hand Hygiene | Perform hand hygiene after touching blood, body fluids, secretions, excretions, and contaminated items; after removing gloves; and between patient contacts. hand hygiene includes both hand-washing with either plain or antimicrobial soap and water or use of alcohol-based products (gels, rinses, foam) that contain an emollient and do not require the use of water. If hands are visibly soiled or contaminated with respiratory secretions, they should be washed with soap (either non-antimocribial or antimicrobial) and water. In the absence of visible soiling of hands, approved alcohol-based products for hand-disinfection are preferred over antimicrobial or plain soap and water because of their superior microbicidal activity, reduced drying of the skin and convenience. |
|
Personal Protective Equipment (PPE) |
-Use when touching blood, body fluids, secretions, excretions and contaminated items; for touching mucous membranes and nonintact (cuts and scrapes) skin. -Use during procedures and patient-care activities which necessitate contact of clothing/exposed skin with blood/body fluids, secretions and excretions is anticipated. -Use during procedures and patient-care activities likely to generate splash or spray of blood, body fluids, secretions and excretions |
| Safe work practices | Avoid touching eyes, nose, mouth, or exposed skin with contaminated hands (gloved or ungloved). Avoid touching surfaces that are not directly related to patient care (e.g., door knobs, keys, light switches) with contaminated gloves and other personal protective equipment. (PPE). |
| Soiled patient-care equipment | Handle in a manner that prevents transfer of microorganisms to oneself, others and environmental surfaces. Wear gloves if visibly contaminated. Perform hand hygiene after handling equipment. |
| Environmental cleaning and disinfection | Use EPA-registered hospital detergent-disinfectant. Follow standard facility procedures for cleaning and disinfecting environmental surfaces. Emphasize cleaning/disinfection of frequently-touched surfaces (e.g., bed rails, phones, lavatory surfaces) |
| Droplet precautions | See www.hhs.gov/pandemicflu/plan/www.cdc.gov/ncidod/hip/ISOLAT/droplet_prec_excerpt.htm. |
If these measures seem excessive, consider that if you contract avian influenza with its present mortality rate, you have a greater than 50% chance of dying.27
Because avian influenza poses both personal and global risks, you must remember to consider this dangerous illness when a patient presents with conjunctivitis and/or flu-like symptoms. Also, stay aware of the recent developments of this illness by visiting the Health Advisory Network (HAN) (www.bt.cdc.gov/documentsapp/HAN/han.asp). This site provides updates and reporting of public health concerns, of which the avian influenza is included. Register for this service via your local health department.
Dr. Pate is a clinical associate professor at the University of Houston College of Optometry. He is an attending O.D. in the Ocular Diagnostic/Medical Eye services and several community clinics where he enjoys working with unusual cases.
1. Centers for Disease Control and Prevention. Stages of a Pandemic. www.cdc.gov/flu/pandemic/phases.htm. (Accessed August 23, 2006)
2. World Health Organization. Ten things you need to know about pandemic influenza. www.who.int/csr/disease/influenza/pandemic10things/en/index.html (Accessed August 23, 2006)
3. The White House. Statement on U.S. Pledge of $334 Million in Global Fight Against Bird Flu. www.whitehouse.gov/news/releases/2006/01/20060118-6.html (Accessed August 23, 2006).
4. The White House. Fact Sheet: President Signs Emergency Funding Bill www.whitehouse.gov/news/releases/2006/06/2006061515.html. (Accessed August 23, 2006).
5. National Institutes of Health, National Institute of Allergy and Infectious Disease. Timeline of human flu pandemics www3.niaid.nih.gov/news/focuson/flu/illustrations/timeline/ timeline.html. (Accessed August 23, 2006)
6. Centers for Disease control and Prevention. Avian Influ-enza (bird flu) transmission of Influenza A viruses between animals and people. www.cdc.gov/flu/avian/geninfo/transmission.htm. (Accessed August 23, 2006).
7. Foucher RAM, Munster V, Wallensten A, et al. Characteri-zation of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J Virol 2005 Mar;79(5):2814-22.
8. UABBioterrerism and Emerging Infections Education. www.bioterrorism.uab.edu/EI/Avian/summary.pdf. (Accessed August 23, 2006)
9. Centers for Disease Control and Prevention. Influenza Viruses. www.cdc.gov/flu/avian/gen-info/flu-viruses.htm. (Accessed August 24, 2006)
10. University of Florida IFAS Extension. Avian Influenza: Hong Kong Outbreak. G. D. Butcher, DVM, Ph.D., R. D. Miles, Ph.D., and A. H. Nilipour. Chronology of events related to the Hong Kong avian influenza outbreak http://edis. ifas.ufl.edu/VM103 (Accessed August 24, 2006).
11. Centers for Disease Control and Prevention. Interim rec-ommendations for infection control in health-care facilities caring for patients with known or suspected avian influenza. www.cdc.gov/flu/avian/professional/infect-control.htm. (Accessed August 23, 2006)
12. Influenza pandemic planning The NZ primary health-care online training course www.guidetools.com/influenza/index. html. (Accessed August 24, 2006).
13. Chan PK. Outbreak of avian influenza A (H5N1) virus infection in Hong Kong in 1997. Clin Infect Dis 2002 May 1;34 Suppl 2:S58-64.
14. World Health Organization (WHO) Avian influenza food safety issues. www.who.int/foodsafety/micro/avian/en/ (Accessed August 24, 2006)
15. World Health Organization epidemic and pandemic alert and response (EPR) www.who.int/csr/disease/avian_influenza/avian_faqs/en/index.html(Accessed August 24, 2006).
16. World Health Organization (WHO). Cumulative number of confirmed human cases of avian influenza A (H5N1) since 28 January 2004. www.who.int/csr/disease/avian_influenza/country/cases_table_2006_08_07/en/index.html. (Accessed August 24, 2006)
17. Yuen KY, Chan PK, Peiris M, et al. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet 1998 Feb14;351(9101):467-71.
18. Tran TH, Nguyen T, Nguyen TD, et al. Avian influenza A (H5N1) in 10 patients in Vietnam. N Engl J Med 2004 Mar 18;350(12):1179-88.
19. Centers for Disease Control and Prevention. Avian influenza infection in humans. www.cdc.gov/flu/avian/geninfo/avian-flu-humans.htm. (Accessed August 24, 2006)
20. Foucher RAM, Schneeberger PM, Rozendaal FW, et al. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc Natl Acad Sci U S A 2004 Feb 3;101(5): 1356-61.
21. Koopmans M, Wilbrink B, Conyn M, et al. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Nether-lands. Lancet 2004 Feb 21;363(9409):587-93.
22. Ontario Ministry of Health and Long-Term Care Important Health Notice. Update on H7 avian influenza virus in British Columbia. www.health.gov.on.ca/english/providers/program/pubhealth/sars/docs/docs3/avian_flu_
notice_040804.pdf. (Accessed August 23, 2006)
23. The Immunization Action Coalition. Vaccine information for the public and health professionals. Influenza vaccine questions and answers. www.vaccineinformation.org/flu/qan davax.asp. (Accessed August 23, 2006)
24. National Strategy for Pandemic Influenza. Chapter 3Federal government response to a pandemic. www.white house.gov/homeland/nspi_implementation_chap03.pdf.
25. Nurkin S, Rahal JJ. Is the clinicians necktie a potential fomite for hospital acquired infections? Presented at Annual Meeting of the American Society for Microbiology, May 24, 2004; New Orleans.
26. Day M. Doctors are told to ditch disease spreading neckties. BMJ 2006 Feb 25;332(7539):442.
27. Centers for Disease Control. Key facts about avian influ-enza (Bird Flu) and avian influenza A (H5N1) virus.www.cdc.gov/flu/avian/gen-info/facts.htm. (Accessed August 24, 2006)

